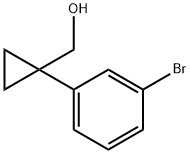
1-(3-BroMophenyl)cyclopropaneMethanol synthesis
- Product Name:1-(3-BroMophenyl)cyclopropaneMethanol
- CAS Number:886366-33-2
- Molecular formula:C10H11BrO
- Molecular Weight:227.1
749928-59-4
29 suppliers
$55.00/250mg

886366-33-2
9 suppliers
inquiry
Yield:886366-33-2 94%
Reaction Conditions:
with diisobutylaluminium hydride in toluene at -78 - 20;
Steps:
5.5C Example 5C: (1 -(3-Bromophenyl)cyclopropyl)methanol
Example 5C: (1 -(3-Bromophenyl)cyclopropyl)methanol j00227j Example 5B (49 mg, 0.192 mmol) was dissolved in toluene (384 .iL) and cooled to -78 °C. DIBAL-H in toluene (960 .iL, 0.960 mmol) was added, and thereaction was warmed to ambient temperature over 4h. The reaction was allowed to stir overnight. The reaction was quenched with MeOH. The reaction was then diluted with 1 N HC1 and extracted thrice with EtOAc. The combined organic extracts were washed with saturated NaHCO3, then brine, dried (Na2504), filtered, and concentrated in vacuo. The crude material was purified by silica gel column chromatography (0 to 100% EtOAcin hexanes) to yield Example SC (41 mg, 94%). 1H NMR (400 MHz, CHLOROFORMc i) ?ppm 7.50(1 H, t, J=1.76 Hz), 7.31 -7.38(1 H, m), 7.21 -7.32(1 H, m), 7.17(1 H, t, J=7.91 Hz), 3.64 (2 H, s), 2.19 (1 H, s), 0.79 -0.91 (4 H, m). 13C NMR (100 MHz, CHLOROFORM-cl) ?ppm 145.2, 132.0, 129.9, 129.6, 127.6, 122.4, 70.4, 27.8, 11.5(2 carbons).
References:
WO2014/201073,2014,A1 Location in patent:Paragraph 00227
150529-73-0
149 suppliers
$6.00/1g

886366-33-2
9 suppliers
inquiry
1878-67-7
324 suppliers
$5.00/5g

886366-33-2
9 suppliers
inquiry
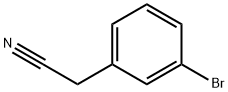
31938-07-5
233 suppliers
$8.00/5g

886366-33-2
9 suppliers
inquiry

124276-83-1
75 suppliers
inquiry

886366-33-2
9 suppliers
inquiry