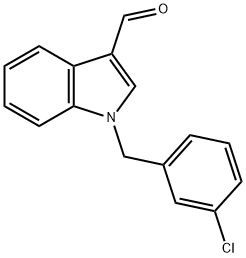
1-(3-CHLOROBENZYL)-1H-INDOLE-3-CARBALDEHYDE synthesis
- Product Name:1-(3-CHLOROBENZYL)-1H-INDOLE-3-CARBALDEHYDE
- CAS Number:90815-01-3
- Molecular formula:C16H12ClNO
- Molecular Weight:269.73
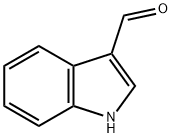
487-89-8
601 suppliers
$6.00/10g
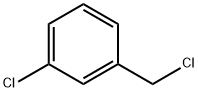
620-20-2
313 suppliers
$5.00/25g

90815-01-3
14 suppliers
$57.50/250mg
Yield:90815-01-3 79%
Reaction Conditions:
with 1,8-diazabicyclo[5.4.0]undec-7-ene in N,N-dimethyl-formamide;Reflux;
Steps:
General Procedure for the Preparation of 3a-3i.
General procedure: Indole-3-carboxaldehyde (1, 5 mmol) was dissolved in appropriate quantity of dimethylformamide (DMF) and 2.4 ml of 1,8-Diazabicycloundec-7-ene was added. Further, 6 mmol appropriate benzyl chloride (2) was added slowly to the mixture and refluxed for >20 hours [21]. The product was extracted by fractionation from ethyl acetate and water. The organic layer was concentrated to give crude product which was then purified using column chromatography (petroleum ether: ethyl acetate).
References:
Chadha, Navriti;Silakari, Om [Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,# 11,p. 2324 - 2330] Location in patent:supporting information