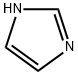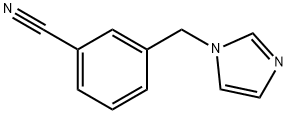
1-(3-Cyanobenzyl)imidazole synthesis
- Product Name:1-(3-Cyanobenzyl)imidazole
- CAS Number:143426-59-9
- Molecular formula:C11H9N3
- Molecular Weight:183.21
Yield:143426-59-9 39.7%
Reaction Conditions:
with potassium carbonate in acetonitrile at 80; for 1 h;
Steps:
12.1 The first step: the preparation of 3-((1H-imidazol-1-yl)methyl)benzonitrile
The raw material 3-(bromomethyl)benzonitrile (14.0g, 71.4mmol, 1.0eq) and 1H-imidazole (5.35g, 78.6mmol, 1.1eq) were dissolved in acetonitrile (140mL), potassium carbonate (29.6g) was added at room temperature g, 214 mmol, 3.0 eq), then the reaction was warmed to 80 °C and stirred for 1 h. After the reaction was detected by LC-MS, the reaction was cooled to room temperature, filtered, and the filter cake was washed with ethyl acetate. After the filtrate was concentrated to remove acetonitrile, water (200 mL) was added, extracted with ethyl acetate (200 mL x 2), and the combined organic phases Dry over anhydrous sodium sulfate, filter and concentrate to obtain crude product. The crude product was purified by flash chromatography (DCM:MeOH=100:0 to 20:1) to give the title compound (5.20 g, 39.7%).
References:
WO2022/161420,2022,A1 Location in patent:Page/Page column 60