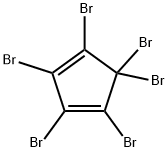
1,3-Cyclopentadiene,1,2,3,4,5,5-hexabromo- synthesis
- Product Name:1,3-Cyclopentadiene,1,2,3,4,5,5-hexabromo-
- CAS Number:14310-17-9
- Molecular formula:C5Br6
- Molecular Weight:539.48
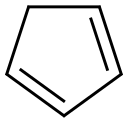
542-92-7
90 suppliers
inquiry

14310-17-9
4 suppliers
inquiry
Yield:14310-17-9 44%
Reaction Conditions:
with potassium hypobromite;potassium hydroxide in water at -5; for 0.166667 h;
Steps:
1,2,3,4-tetrabromo-5,5-dimethoxycyclopenta-1,3-diene (5)
Title compound was obtained from a literatureprocedure using hexabromo cyclopentadiene (4) and sodium methoxide. Hexabromo cycopentadiene was obtained froma modified literature procedure using cycopentadiene and KOBr. Doubly distilled cycopentadiene (2 g, 3.03 mmol) was added to an aqueous solution (227 mL) containing KOBr (0.8 M) and KOH (2.1 M) at -5°C while vigorously stirring. A heavy yellow solid was precipitated after 10 min. The precipitate was filtered and recrystallized in hexanes to yield yellow crystals of hexabromocyclopentadiene (7.2g, 44%). Added 2.67 mL of NaOMe (30% in methanol, 14.458 mmol) to a solution containing hexabromocyclopentadiene (3.9g, 7.229 mmol) in diethylene glycol dimethyl ether (20 mL) at -78°C. Then the solution was allowed to reach room temperature and stirred for about 3 hours and then poured onto chopped ice (200 mL). After the ice had melted, the mixture was extracted with CH2Cl2 (3 × 30 mL) and the combined organic extracts were dried (Na2SO4) and evaporated. The crude product was purified by column chromatography to yield titled compound 5.
References:
Mahendran, Adaickapillai;Gopinath, Purushothaman;Breslow, Ronald [Tetrahedron Letters,2015,vol. 56,# 33,p. 4833 - 4835] Location in patent:supporting information
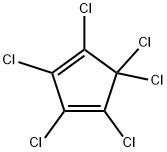
77-47-4
2 suppliers
$330.00/50g

14310-17-9
4 suppliers
inquiry