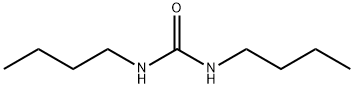
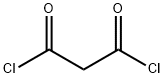
1,3-Dibutyl-pyriMidine-2,4,6-trione synthesis
- Product Name:1,3-Dibutyl-pyriMidine-2,4,6-trione
- CAS Number:5770-40-1
- Molecular formula:C12H20N2O3
- Molecular Weight:240.3
Yield:5770-40-1 93%
Reaction Conditions:
with acetic anhydride in acetic acid at 80 - 90; for 2.5 h;
Steps:
1.a
The synthesis of barbituric acid compounds has been described (Biltz, H. , Wittek, H. Ber. 1921,54B, 1035-58; Carnell, A. J. et. al. Tetrahedron Lett. 1999,40, 8029-8032). Thus, to a mixture of 30 g (0.174 mol) N, N'-dibutylurea and 18.2 g (0.174 mol) malonic acid in 200 mL of Acetic acid was added 62.2 g (0.61 mol) of acetic anhydride. The mixture was heated to 80- 90 °C with stirring for 2.5 h. The resulting solution was concentrated under vacuum to a brown oil which was flushed through a short plug of silica gel (1 kg) eluting with 5%-50% EtOAc in hexanes to give a yellow colored oil (38.8 g, 93%). Upon standing the oil solidified to the pale yelow solid intermediate 1, 3-dibutylpyrimidine-2, 4,6 (1H, 3H, 5trione of sufficient purity to carry forward into the next reaction.
References:
WO2005/77918,2005,A1 Location in patent:Page/Page column 12-13

161494-64-0
0 suppliers
inquiry

5770-40-1
14 suppliers
inquiry
111-36-4
201 suppliers
$41.00/5ml

5770-40-1
14 suppliers
inquiry