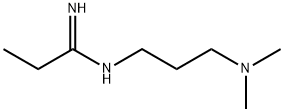
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide synthesis
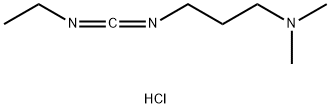
- Product Name:1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide
- CAS Number:1892-57-5
- Molecular formula:C8H17N3
- Molecular Weight:155.24
25952-53-8
782 suppliers
$9.00/25g

1892-57-5
302 suppliers
$5.00/1g
Yield:-
Reaction Conditions:
with potassium carbonate in trifluoromethylbenzene (BTF);water;
Steps:
1
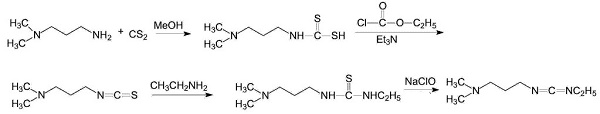
Example 1 Synthesis of Cabergoline N-(3-Dimethylaminopropyl)-N-ethyl carbodiimide hydrochloride (EDAC.HCl) is added to a stirred mixture of trifluoromethylbenzene (BTF) and 25% w/w aqueous potassium carbonate. The mixture is stirred until a clear two-phase solution is obtained. The layers are allowed to separate and the lower aqueous layer is discarded. The upper organic layer is stirred with anhydrous potassium carbonate and filtered to provide a solution of EDAC in BTF. A suspension of a compound of formula (II) in BTF is stirred at IS to 24° C. and the required quantity of EDAC in BTF solution is charged. The resulting suspension is then heated to a temperature of 35 to 38° C. and maintained at this temperature until the reaction is complete. The solution is filtered and purified water added. Glacial acetic acid is then added to bring the pH to 5.0 to 5.5. The upper aqueous phase is separated. t-Butyl methyl ether is added to the upper aqueous phase and a 20% w/w potassium hydroxide solution is added to adjust the mixture to pH 9.5 to 10.0. The layers are separated and the lower aqueous layer is extracted with t-butyl methyl ether. The two upper organic layers are combined and washed with 13% aqueous sodium chloride. The upper organic layer is then separated and stirred with charcoal. The mixture is then filtered and concentrated under vacuum at 35 to 38° C. to about 2 to 3 volumes. Acetonitrile is added and the solvent exchanged via distillation under vacuum at 35 to 38° C. to about 2 to 3 volumes. The resulting acetonitrile solution may then be used as starting material for producing desired polymorphs for incorporation into pharmaceutical preparations, e.g. by the procedures of the above-mentioned international Patent Application No. WO 05/105796 and UK Patent Application Nos. 0505965.4 and 0515430.7.
References:
US2007/197576,2007,A1 Location in patent:Page/Page column 4
32897-26-0
95 suppliers
$53.96/250mg

1892-57-5
302 suppliers
$5.00/1g
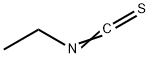
542-85-8
209 suppliers
$46.00/25mL
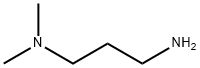
109-55-7
341 suppliers
$14.00/25mL

1892-57-5
302 suppliers
$5.00/1g
![[3-(Dimethylamino)propyl]dithiocarbamic acid](/CAS/GIF/18997-72-3.gif)
18997-72-3
0 suppliers
inquiry

1892-57-5
302 suppliers
$5.00/1g
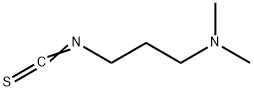
27421-70-1
36 suppliers
$265.00/250mg

1892-57-5
302 suppliers
$5.00/1g