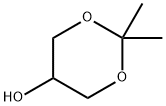
1,3-Dioxan-5-ol, 2,2-dimethyl- synthesis
- Product Name:1,3-Dioxan-5-ol, 2,2-dimethyl-
- CAS Number:3391-30-8
- Molecular formula:C6H12O3
- Molecular Weight:132.16
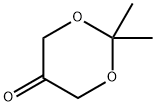
74181-34-3
132 suppliers
$13.00/100mg

3391-30-8
26 suppliers
$250.00/500mg
Yield:3391-30-8 93.4%
Reaction Conditions:
Stage #1:2,2,-dimethyl-1,3-dioxan-5-one with lithium aluminium tetrahydride in diethyl ether at 0 - 8; for 1 h;
Stage #2: with sodium hydroxide in diethyl ether;water at 0 - 10;
Steps:
9.a
(9a) 2,2-dimethyl-1,3-dioxan-5-ol To a diethyl ether (150 ml) solution of 2,2-dimethyl-1,3-dioxan-5-one (15 g, 0.115 mol), lithium aluminum hydride (4.38 g, 0.115 mol) was added at 0 to 8° C. for one hour in a nitrogen atmosphere. To the reaction mixture, water (4.2 ml), a 5N aqueous sodium hydroxide solution (4.2 ml), and water (12.8 ml) were sequentially added dropwise at 0 to 10° C. The mixture was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to obtain the title compound (14.2 g, 93.4%) as a colorless oil. 1H NMR(400 MHz, CDCl3) δppm; 1.44(3H, s), 1.46(3H, s), 2.75-2.95(1H, br), 3.51-3.55(1H, m), 3.74-3.79(2H, m), 4.05-4.10(2H, m).
References:
Eisai Co., Ltd. US2007/10542, 2007, A1 Location in patent:Page/Page column 33
67-64-1
6 suppliers
$17.30/10ml
56-81-5
1640 suppliers
$5.00/25g

3391-30-8
26 suppliers
$250.00/500mg
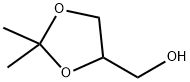
100-79-8
364 suppliers
$9.00/25g
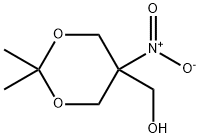
4728-14-7
12 suppliers
inquiry

3391-30-8
26 suppliers
$250.00/500mg
![(5-AMINO-2,2-DIMETHYL-[1,3]DIOXAN-5-YL)-METHANOL](/CAS/GIF/53104-32-8.gif)
53104-32-8
43 suppliers
$60.00/50mg

3391-30-8
26 suppliers
$250.00/500mg
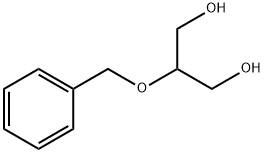
14690-00-7
135 suppliers
$70.00/250mg

77-76-9
389 suppliers
$9.00/5ml

3391-30-8
26 suppliers
$250.00/500mg