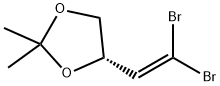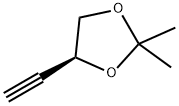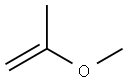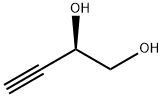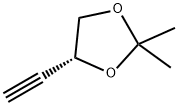
1,3-Dioxolane, 4-ethynyl-2,2-dimethyl-, (R)- (9CI) synthesis
- Product Name:1,3-Dioxolane, 4-ethynyl-2,2-dimethyl-, (R)- (9CI)
- CAS Number:186521-82-4
- Molecular formula:C7H10O2
- Molecular Weight:126.15
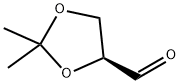
22323-80-4
140 suppliers
$71.50/5g

186521-82-4
7 suppliers
inquiry
Yield:186521-82-4 70%
Reaction Conditions:
with dimethyl 1-(1-diazo-2-oxopropyl)phosphonate;potassium carbonate in methanol at 0 - 20; for 12.5 h;
Steps:
17
Example 17 (R)-(-)-4-Ethynyl-2,2-dimethyl-1 ,3-dioxolaneThe solution of L-glyceraldehyde acetonide prepared from ascorbic acid (Ch. Hubschwerlin, Synthesis 1986. 962-964) (1.03 g, 7.90 mmol) and Bestmann-Ohira reagent (2.42 g, 12.6 mmol) in methanol (50 ml) is cooled to 00C. Potassium carbonate (2.33 g, 16.8 mmol) is then added portionwise during 30 min, followed by stirring the reaction mixture at room temperature for 12 hours. Then a saturated solution of NH4CI (50 ml) is added and the resulting solution is extracted with pentane (2 x 250 ml). The combined organic layers are dried over magnesium sulfate and after removal of the solvent the residue is subjected to silica gel chromatography (pentane/diethyl ether 10:1 ) to afford 0.7 g (70%) of (R)-(-)-4- ethynyl-2 ,2-dimethyl-1 ,3-dioxolane in the form of yellow oil.[α]D -40° (c 1.2, CH2CI2). IR (film): 2121 cm"1.1H NMR (200 MHz, CDCI3), δ: 4.67 (1 H, m), 4.15 (1 H, dd, J 8.1. 6.4 Hz), 3.91 (1 H, dd, J 8.1. 6.2 Hz), 2.47 (1 H, d, J 2.3 Hz), 1.47 (3H, s, Me), 1.35 (3H, s, Me).
References:
WO2010/97350,2010,A1 Location in patent:Page/Page column 29-30
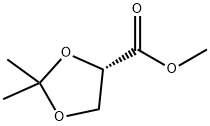
60456-21-5
104 suppliers
$40.00/1/ G

186521-82-4
7 suppliers
inquiry
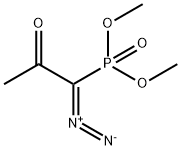
90965-06-3
207 suppliers
$30.25/1g

22323-80-4
140 suppliers
$71.50/5g

186521-82-4
7 suppliers
inquiry