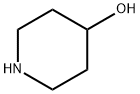
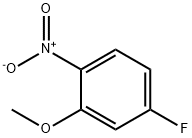
1-(3-Methoxy-4-nitrophenyl)piperidin-4-ol synthesis
- Product Name:1-(3-Methoxy-4-nitrophenyl)piperidin-4-ol
- CAS Number:761440-22-6
- Molecular formula:C12H16N2O4
- Molecular Weight:252.27
Yield:761440-22-6 99%
Reaction Conditions:
with potassium carbonate in dimethyl sulfoxide at 20; for 24 - 72 h;Product distribution / selectivity;
Steps:
B37; B38.A
Intermediate B37: 1-[4-amino-3-(methyloxy)phenyl]-4-piperidinol; A solution of 4-fluoro-2-(methyloxy)-1 -nitrobenzene (3.0 g, 17.5 mmol), 4- hydroxypiperidine (1.77 g, 17.5 mmol) and potassium carbonate (2.9 g, 21 mmol) in DMSO was stirred for 24 h. The reaction was diluted with water and extracted three times with Ethyl acetate. The combined organic solutions were dried over MgSO4 and concentrated. The resulting residue was stirred in ethanol and acetic acid with 10% Pd/C under 30 psi of hydrogen for 16 hr. The reaction was filtered through a pad of celite, rinsed with Ethyl acetate. The filtrate was concentrated and dissolved in methylene chloride. The organic solution was washed with saturated sodium bicarbonate, dried over MgSO4, and concentrated to give 1-[4-amino-3- (methyloxy)phenyl]-4-piperidinol (2.44 g, 63% yield over 2 steps). 1H NMR (400 MHz, de-DMSO) δ 6.49-6.45 (m, 2H), 6.27 (dd, J = 8.4 and 2.4 Hz, 1 H), 4.60 (d, J = 4.0 Hz, 1 H), 4.18 (s, 2H), 3.71 (s, 3H), 3.54-3.48 (m, 1 H), 3.25-3.22 (m, 2H), 2.63-2.56 (M, 2H), 1.80-1.76 (m, 2H), 1.51-1.42 (m, 2H).; Intermediate B38: 4-(3,3-difluoro-1,4'-bipiperidin-1'-yl)-2-(methyloxy)aniline; Step A/Intermediate B39: 1-[3-(methyloxy)-4-nitrophenyl]-4-piperidinol; A mixture of 4-fluoro-2-(methyloxy)-1 -nitrobenzene (7.52 g, 44 mmol), 4- hydroxypiperidine (4.45 g, 44 mmol) and potassium carbonate in 100 ml. DMSO was stirred for 72h. The reaction was diluted with water and extracted twice with Ethyl acetate. The combined organic layers were washed with brine, dried over MgSO4, and concentrated to give 1-[3-(methyloxy)-4-nitrophenyl]-4-piperidinol (10.9 g, 99% yield). 1H NMR (400 MHz, d6-DMSO) δ 7.84 (d, J = 9.2 Hz, 1 H), 6.54 (d, J = 9.6 Hz, 1 H), 6.46 (s, 1 H), 4.73 (d, J = 4.4 Hz, 1 H), 3.86 (s, 3H), 3.80-3.69 (m, 3H), 3.18-3.12 (m, 2H), 1.79-1.76 (m, 2H), 1.42-1.34 (m, 2H).
References:
WO2009/20990,2009,A1 Location in patent:Page/Page column 96-97