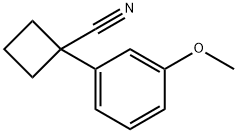
1-(3-METHOXYPHENYL)CYCLOBUTANECARBONITRILE synthesis
- Product Name:1-(3-METHOXYPHENYL)CYCLOBUTANECARBONITRILE
- CAS Number:74205-15-5
- Molecular formula:C12H13NO
- Molecular Weight:187.24
Yield:74205-15-5 44.2%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide at 0 - 20; for 17 h;
Steps:
10.1 Synthesis of 1-(3-methoxyphenyl)cyclobutane-1-carbonitrile
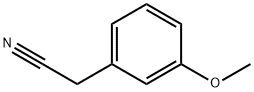
2-(3-methoxyphenyl)acetonitrile (5.000 g, 33.972 mmol) and sodium hydride (60.00%, 2.989 g, 74.738 mmol) in N,N-dimethylformamide (100 mL) at 0 °C to this mixture 1,3-dibromopropane (3.447 mL, 33.972 mmol) was added and stirred at room temperature for 17 hours, a saturated aqueous sodium bicarbonate solution (10 mL) was added to the reaction mixture at room temperature, And the reaction was terminated. The solvent was removed from the reaction mixture under reduced pressure, the obtained concentrate was poured into water and extracted with ethyl acetate. The organic layer was washed with a saturated aqueous sodium chloride solution, and water was removed with anhydrous magnesium sulfate, followed by filtration and concentration under reduced pressure. The concentrate was purified by column chromatography (SiO2, 40 g cartridge;ethyl acetate/hexane=0% to 5%) and the title compound (2.810 g, 44.2%) was obtained as a colorless oil.
References:
KR2017/43091,2017,A Location in patent:Paragraph 0317-0320