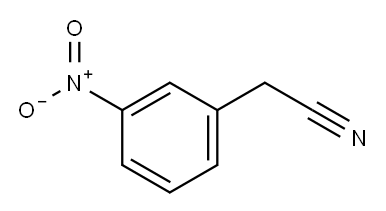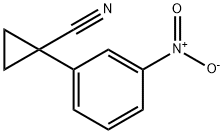
1-(3-Nitro-phenyl)-cyclopropanecarbonitrile synthesis
- Product Name:1-(3-Nitro-phenyl)-cyclopropanecarbonitrile
- CAS Number:124276-69-3
- Molecular formula:C10H8N2O2
- Molecular Weight:188.18
Yield:124276-69-3 635 mg
Reaction Conditions:
with sodium hydride in dimethyl sulfoxide;mineral oil at 20; for 16 h;
Steps:
1 Step 1: 1 -(3-Nitrophenyl)cyclopropanecarbonitrile
To a stirred solution of 2-(3-nitrophenyl)acetonitrile (500 mg, 3.08 mmol) in DMSO (10 mL) were added 1,2-dibromoethane (266 jiL, 3.08 mmol) and sodium hydride (60% w/w, 123 mg, 3.08 mmol) at RT. The mixture stirred at RT for 16 h. The reaction mixture was quenched with aqueous sodium sulfate solution, diluted with ethyl acetate and the organic layer was washed with saturated sodium bicarbonate solution followed by brine. The organic layer was dried over anhydrous sodium, filtered and concentrated under reduced pressure. The residue obtained was purified by column chromatography to yield 635 mg of the desired product. ‘HNMR (400 MHz, DMSO-d6) ? 1.69 (t, J 2.8 Hz, 2H), 1.87 (t, J= 2.8 Hz, 2H), 7.70 (t, J=8.0 Hz, 1H), 7.78-7.80 (m, 1H), 8.17-8.19 (m, 2H).
References:
WO2018/215668,2018,A1 Location in patent:Page/Page column 79; 80
619-25-0
212 suppliers
$8.00/5g

124276-69-3
7 suppliers
inquiry
3958-57-4
238 suppliers
$14.00/10g

124276-69-3
7 suppliers
inquiry