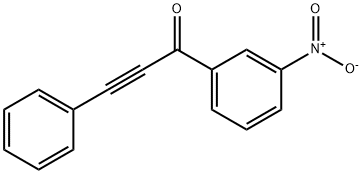
1-(3-nitrophenyl)-3-phenyl-2-propyn-1-one synthesis
- Product Name:1-(3-nitrophenyl)-3-phenyl-2-propyn-1-one
- CAS Number:16616-39-0
- Molecular formula:C15H9NO3
- Molecular Weight:251.24
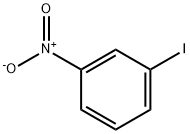
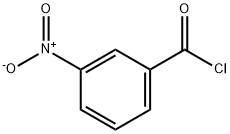
645-00-1
9 suppliers
$50.00/1g
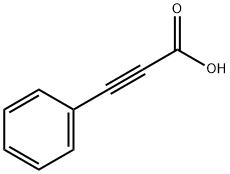
201230-82-2
1 suppliers
inquiry
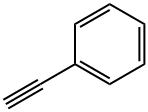
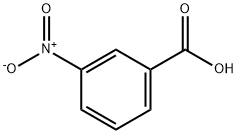
536-74-3
295 suppliers
$10.00/1g

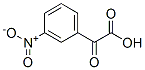
16616-39-0
17 suppliers
inquiry
Yield:16616-39-0 98 %Chromat.
Reaction Conditions:
with bis-[1-(5’-diphenylphosphinothiazol-2’-yl)-imidazolyl]dichloropalladium(II);triethylamine in N,N-dimethyl-formamide at 90; under 7500.75 Torr; for 1.5 h;Autoclave;
Steps:
General procedure for carbonylative Sonogashira reaction
General procedure: In a typical experiment, the isolated crystalline precatalyst 1A (or 2A, 0.005 mmol) was sequentially mixed with 3 mL of solvent (DMF or [Bmim]PF6 if required), iodobenzene (5mmol), phenylacetylene (6 mmol), and Et3N (7.5 mmol). The obtained mixture was placed in a sealed Teflon-lined stainless steel autoclave, purged with syngas (CO, 1.0 MPa) and then stirred vigorously at the required temperature for the appointed time. Upon completion of the reaction, the mixture wasc ooled to room temperature and the pressure was carefully released. The reaction mixture was extracted with diethyl ether(5 mL 3). The ether fractions were combined and then analyzedby GC to determine the conversion of PhI (1-dodecane as an internal standard) and the selectivity for the carbonylative products (normalization method). The structures of the carbonylative products were further confirmed by GC-MS.
References:
Yang, Da;Wang, Dongliang;Liu, Huan;Zhao, Xiaoli;Lu, Yong;Lai, Shijun;Liu, Ye [Cuihua Xuebao/Chinese Journal of Catalysis,2016,vol. 37,# 3,p. 405 - 411]

88596-60-5
0 suppliers
inquiry

16616-39-0
17 suppliers
inquiry