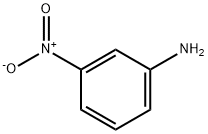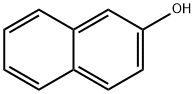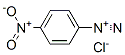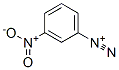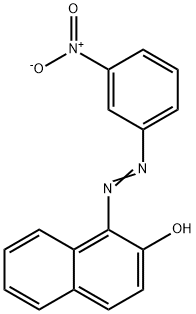
1-[(3-nitrophenyl)azo]-2-naphthol synthesis
- Product Name:1-[(3-nitrophenyl)azo]-2-naphthol
- CAS Number:6471-46-1
- Molecular formula:C16H11N3O3
- Molecular Weight:293.28
Yield:6471-46-1 97%
Reaction Conditions:
Stage #1: 3-nitro-anilinewith hydrogenchloride in water at 0 - 20; for 0.0833333 h;
Stage #2: β-naphthol in water at 0 - 5;
Steps:
7.1 2.6 General procedure for the synthesis of azo dyes 3a-t
General procedure: Aniline derivative was added to a vessel containing HCl 37% (0.5ml) and the mixture was stirred in ice bath at 0-5°C. Then, NFSPI 10 was added to the mixture and grinded at room temperature for the times shown in Table2 to produce the related diazonium salt. The phenolate compound was added slowly to the mixture to produce the desired azo dye at 0-5°C. After completion of the reaction, ethyl acetate was added to the reaction mixture and stirred thoroughly. Then, poly ionic compound 9 was filtered off and the crude azo dye was isolated by evaporation of ethyl acetate. For more purification, crude product was recrystallized from ethanol/H2O. The products were characterized by their FT-IR, 1H-NMR and 13C-NMR spectra.
References:
Valizadeh, Hassan;Shomali, Ashkan;Ghorbani, Jalal;Noorshargh, Saeideh [Dyes and Pigments,2015,vol. 117,p. 64 - 71]

99779-37-0
0 suppliers
inquiry
![1-[(3-nitrophenyl)azo]-2-naphthol](/CAS/GIF/6471-46-1.gif)
6471-46-1
3 suppliers
inquiry
![2-Naphthalenamine, 1-[2-(3-nitrophenyl)diazenyl]-](/CAS/20210305/GIF/10147-94-1.gif)
10147-94-1
0 suppliers
inquiry
![1-[(3-nitrophenyl)azo]-2-naphthol](/CAS/GIF/6471-46-1.gif)
6471-46-1
3 suppliers
inquiry