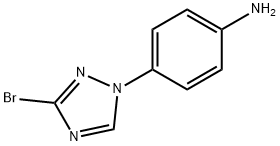
1-(4-AMINO-PHENYL)-3-BROMO-1,2,4-TRIAZOLE synthesis
- Product Name:1-(4-AMINO-PHENYL)-3-BROMO-1,2,4-TRIAZOLE
- CAS Number:1129540-72-2
- Molecular formula:C8H7BrN4
- Molecular Weight:239.07
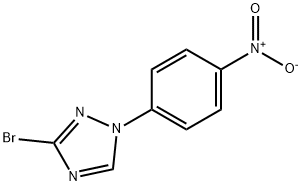
1129540-71-1
18 suppliers
inquiry

1129540-72-2
20 suppliers
$60.00/5mg
Yield:1129540-72-2 94%
Reaction Conditions:
with iron;ammonium chloride in ethanol;water at 80;
Steps:
94 Example 94: Preparation of 4-(3-bromo-1H-1,2,4-triazol-1-yl)aniline (C490)
3-Bromo-1-(4-nitrophenyl)-1H-1,2,4-triazole (C486; 4.00 g, 14.9 mmol) was suspended in ethanol (59.5 mL) and water (14.87 mL), and to the resultingsuspension were added ammonium chloride (3.18 g, 59.5 mmol) and iron (3.32 g, 59.5 mmol). The flask was outfitted with arefluxcondenser and placed on a preheated plate set to 80 °C and allowed to stir at that temperature until LC-MS indicated complete product formation (0.5-2 h). The reaction mixture was cooled to room temperature, diluted with EtOAc, and filtered through a pad of diatomaceous earth, washing with EtOAc and water. The filtrate was transferred to a separatory funnel with the aid of water, and the layers were separated. The aqueous layer was extracted with EtOAc. The combined organic layers were washed with brine, dried over Na2SO4-magnesium sulfate, and concentrated in vacuo to afford the title compound as a beige solid (3.36 g, 94%), which required no further purification:1H NMR (400 MHz, CDCl3) d 8.26 (s, 1H), 7.40- 7.34 (m, 2H), 6.79- 6.71 (m, 2H), 3.88 (s, 2H); ESIMS m/z 239 ([M+H]+).
References:
WO2021/11722,2021,A1 Location in patent:Page/Page column 324-325