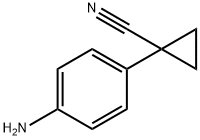
1-(4-Aminophenyl)cyclopropanecarbonitrile synthesis
- Product Name:1-(4-Aminophenyl)cyclopropanecarbonitrile
- CAS Number:108858-86-2
- Molecular formula:C10H10N2
- Molecular Weight:158.2

408328-42-7
33 suppliers
inquiry

108858-86-2
22 suppliers
inquiry
Yield:108858-86-2 90%
Reaction Conditions:
with iron;ammonium chloride in ethanol;water at 50; for 0.75 h;Inert atmosphere;
Steps:
8 1-(4-morpholinophenyl)cyclopropanecarbonitrile
To a mixture of 18ml of concentrated H2SO4 and 18ml of 65% HNO3 solution, cooled on an ice bath and under a nitrogen atmosphere, was added neat 1-phenylcyclopropanecarbonitrile (10.00g, 69.84mmol). The reaction mixture was stirred for 15 minutes on ice, warmed to room temperature and stirred for an additional 30 minutes after which TLC (25% EtOAc in PE (40-60)) indicated complete consumption of the starting material. The reaction mixture was poured in water, the solids were filtered, washed with water, dried and recrystallized from EtOH to give 1-(4-nitrophenyl)cyclopropanecarbonitrile (9.47g, 50.32mmol, 72%). 1H NMR (400 MHz, CDCl3) δ ppm 8.21 (d, 2H), 7.43 (d, 2H), 1.91 (m, 2H), 1.54 (m, 2H). To a mixture of the above nitro compound (2.95g, 15.96mmol) in 50ml EtOH and 50ml water containing NH4Cl (8.52g, 159.6mmol) at 50°C under a nitrogen atmosphere was added portionwise iron powder (4.45g, 79.65mmol). The resulting reaction mixture was stirred for 45 minutes after which TLC (25% EtOAc in PE (40-60)) indicated complete consumption of the starting material. The reaction mixture was cooled, filtered over celite and the filter cake was washed with EtOH. The filtrate was evaporated and the residue was partitioned between EtOAc and water. The aqueous layer was extracted with EtOAc and the combined organic layers were washed with brine, dried over MgSO4 and evaporated to give 1-(4-aminophenyl)cyclopropanecarbonitrile (2.27g, 14.35mmol, 90%). 1H NMR (400 MHz, CDCl3) δ ppm 7.09 (d, 2H), 6.64 (d, 2H), 3.70 (bs, 2H), 1.60 (m, 2H), 1.28 (m, 2H). A mixture of the above amine (2.25g, 14.22mmol), 1-chloro-2-(2-chloroethoxy)ethane (2.44g, 17.07mmol), sodium iodide (5.33g, 35.56mmol) and K2CO3 (3.93g, 28.45mmol) in 25ml NMP was stirred for 3h at 120°C under a nitrogen atmosphere after which TLC (25% EtOAc in PE (40-60)) indicated complete consumption of the starting material. The residue was partitioned between EtOAc and water. The aqueous layer was extracted with EtOAc and the combined organic layers were washed with brine, dried over MgSO4 and evaporated. The residue was purified by column chromatography (silica, 5% EtOAc in DCM) to give 1-(4-morpholinophenyl)-cyclopropanecarbonitrile (2.60g, 11.39mmol, 80%) as a crystalline solid. NMR (400 MHz, CDCl3) δ ppm 7.21 (d, 2H), 6.87 (d, 2H), 3.85 (d, 4H), 3.15 (d, 4H), 1.64 (m, 2H), 1.30 (m, 2H).
References:
EP3811945,2021,A1 Location in patent:Paragraph 0064; 0075

915020-91-6
8 suppliers
inquiry

108858-86-2
22 suppliers
inquiry
555-21-5
43 suppliers
$21.00/25g

108858-86-2
22 suppliers
inquiry
50685-26-2
71 suppliers
inquiry

108858-86-2
22 suppliers
inquiry
935-44-4
115 suppliers
$7.00/1g

108858-86-2
22 suppliers
inquiry