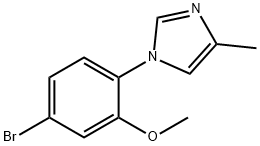
1-(4-BROMO-2-METHOXYPHENYL)-4-METHYL-1H-IMIDAZOLE synthesis
- Product Name:1-(4-BROMO-2-METHOXYPHENYL)-4-METHYL-1H-IMIDAZOLE
- CAS Number:870838-56-5
- Molecular formula:C11H11BrN2O
- Molecular Weight:267.12

958245-18-6
44 suppliers
$119.00/100mg

870838-56-5
14 suppliers
$245.43/5g
Yield:870838-56-5 91.3%
Reaction Conditions:
Stage #1: 3-methoxy-4-(4-methyl-1H-imidazol-1-yl)anilinewith sulfuric acid;acetic acid;sodium nitrite at 10 - 20; for 0.5 h;
Stage #2: with hydrogen bromide;copper(I) bromide in water at 20; for 1 h;
Stage #3: with sodium carbonate in dichloromethane;water;
Steps:
A.A2.c
c) Preparation of intermediate 5 A stirred sol. OfNaNO2 (7.47 g, 108 mmol) in cone. H2SO4 (160 ml) was cooled to 10 0C. A sol. of intermediate 4 (20.0 g, 98.4 mmol) in AcOH (200 ml) was added at such a rate that the temperature of the r.m. was maintained below 10 0C. After addition was completed, the ensuing r.m. was stirred at r.t. for 30 min. This sol. was added, dropwise, to a stirred sol. of CuBr (28.2 g, 196.8 mmol) in 48% HBr (200 ml) at r.t.The ensuing r.m. was stirred for 1 h, then diluted with 1 1 ice/H2O. The resulting white precipitate was collected by filtration and washed with H2O. (The mother liquor was treated as described below). The solid was then suspended in DCM/sat. aq. Na2Cθ3 sol. and the resulting slurry filtered over dicalite. The o.l. of the filtrate was washed with diluted NH4OH until the disappearance of blue colour. The organic phase was dried (MgSO/i), filtered and evaporated to give a brown solid. The mother liquor was basifed with solid Na2CC^, then extracted with DCM. The combined organic extracts were washed with diluted NH4OH until the disappearance of blue colour. The organic phase was dried (MgSOzi), filtered and evaporated to give a brown solid which was combined with the previously obtained solid. Yield: 24.0 g of intermediate 5 (91.3%).
References:
WO2011/6903,2011,A1 Location in patent:Page/Page column 117-118
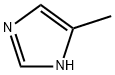
822-36-6
486 suppliers
$9.00/1g
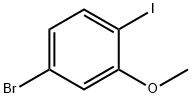
791642-68-7
116 suppliers
$10.00/250mg

870838-56-5
14 suppliers
$245.43/5g

1145786-43-1
0 suppliers
inquiry

870838-56-5
14 suppliers
$245.43/5g

870838-55-4
0 suppliers
inquiry

870838-56-5
14 suppliers
$245.43/5g

958245-17-5
22 suppliers
inquiry

870838-56-5
14 suppliers
$245.43/5g