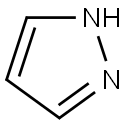
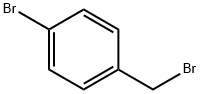
1-(4-BROMOBENZYL)-1H-PYRAZOLE synthesis
- Product Name:1-(4-BROMOBENZYL)-1H-PYRAZOLE
- CAS Number:1159826-63-7
- Molecular formula:C10H9BrN2
- Molecular Weight:237.1
Yield:1159826-63-7 95%
Reaction Conditions:
Stage #1: 1H-pyrazolwith 18-crown-6 ether;potassium tert-butylate in diethyl ether at 20; for 0.25 h;
Stage #2: 1-bromomethyl-4-bromobenzene in diethyl ether; for 1.5 h;
Steps:
142.1
(1) 1-(4-Bromobenzyl)-1H-pyrazole Potassium tert-butoxide (1.4 g, 12 mmol) and 18-crown-6-ether (0.26 g, 1.0 mmol) were dissolved in diethyl ether (25 mL), and pyrazole (0.68 g, 10 mmol) was added, followed by stirring at room temperature for 15 minutes. A solution of 1-bromo-4-(bromomethyl)benzene in diethyl ether (25 mL) was added to the reaction solution, followed by stirring for 1.5 hours. Water was added to the reaction solution, followed by extraction with ethyl acetate. After the organic layer was concentrated under reduced pressure, the resulting residue was purified by silica gel column chromatography (Moritex Corporation, elution solvent:hexane/ethyl acetate), and a fraction corresponding to the Rf value=0.40 (hexane/ethyl acetate=2/1) by thin layer chromatography was concentrated under reduced pressure to afford the title compound (2.2 g, 9.5 mmol) as a colorless oil (yield 95%). 1H-NMR (500 MHz, CDCl3) δ: 7.57-7.54 (1H, m), 7.45 (2H, d, J=8 Hz), 7.42-7.36 (1H, m), 7.08 (2H, d, J=8 Hz), 6.32-6.27 (1H, m), 5.28 (2H, s).
References:
US2011/112103,2011,A1 Location in patent:Page/Page column 73
106-38-7
391 suppliers
$14.00/5g

1159826-63-7
21 suppliers
inquiry