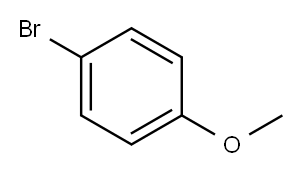
1-(4-bromobutyl)-4-methoxybenzene synthesis
- Product Name:1-(4-bromobutyl)-4-methoxybenzene
- CAS Number:35191-43-6
- Molecular formula:C11H15BrO
- Molecular Weight:243.14
Yield:35191-43-6 57%
Reaction Conditions:
Stage #1: 1-bromo-4-methoxy-benzenewith magnesium in tetrahydrofuran; for 3 h;Heating / reflux;
Stage #2: 1,4-dibromo-butane;lithium chloride;copper dichloride in tetrahydrofuran at 0 - 20; for 13 h;
Steps:
1.i
After starting the Grignard reaction by adding 5.00 ml 4-bromoanisole to a mixture of 4.86 g (0.20 mol) magnesium turnings and 100 ml THF, 20.00 ml 4-bromoanisole (total: 25.0 ml (37.4 g; 0.20 mol) were added at a pace sufficient to maintain reflux temperature. The reaction mixture was heated to reflux for additional 3 h, cooled to r.t. and dropped at 0° C. within 1 h to a stirred solution prepared by mixing 129.6 g (71.6 ml, 0.60 mol) 1,4-dibromo-butane in 200 ml THF with a freshly prepared solution of 0.17 g (4.0 mmol) LiCl and 0.267 g (2.0 mmol) Cu(II)Cl2 in 20 ml THF. Stirring was continued for 12 h at r.t. followed by the addition of 100 ml of a 20% ammonium chloride solution and 200 ml ethyl acetate. The water phase was extracted twice with 50 ml ethyl acetate, all organic phases were combined, dried over sodium sulphate and evaporated. The resulting oil was fractionated by vacuum distillation. Yield: 27.7 g (57%), b.p. 112-115° C./0.15 mbar.
References:
US2005/197370,2005,A1 Location in patent:Page/Page column 13
52244-70-9
111 suppliers
$6.00/1g

35191-43-6
6 suppliers
inquiry
4521-28-2
199 suppliers
$9.00/1g

35191-43-6
6 suppliers
inquiry
88537-45-5
0 suppliers
inquiry

35191-43-6
6 suppliers
inquiry
81786-51-8
0 suppliers
inquiry

35191-43-6
6 suppliers
inquiry