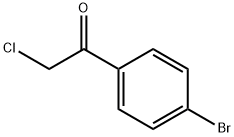
1-(4-bromophenyl)-2-chloroethan-1-one synthesis
- Product Name:1-(4-bromophenyl)-2-chloroethan-1-one
- CAS Number:4209-02-3
- Molecular formula:C8H6BrClO
- Molecular Weight:233.49
Yield: 55% , 15%
Reaction Conditions:
with [2,2]bipyridinyl;copper(l) chloride in 1,2-dichloro-ethane;benzene for 1.5 h;Inert atmosphere;Schlenk technique;Reflux;diastereoselective reaction;
Steps:
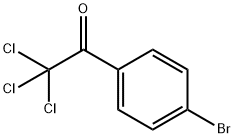
Reaction of 2,2,2-trichloro-1-phenylethanone (1a-k) with CuCl/bpy:
General procedure: A two-neck round bottom flask fitted with a rubber septum was connected to a Schlenk tube through a condenser. The flask was evacuated and dried by heating with a flame while the vacuum pump was turned on, and then the flask was filled with dry nitrogen. After removing the rubber septum, it was charged with CuCl (800 mg, 8 mmol), 2,2’-bipyridyl (1.248 gm, 8 mmol), and a magnetic bar under continuous flow of nitrogen. The rubber septum was then restored and the flask was evacuated and filled with nitrogen. Dry Benzene (10 ml) was then injected into the flask to ensure the formation of the brown colored copper-bpy complex. Now the solution of 2,2,2-trichloro-1-phenylethanone 1 (4 mmol) in DCE (10 ml) was injected through the septum. The flask was again evacuated and filled with nitrogen after cooling it in an ice bath. The ice bath was removed and the reaction mixture was heated at reflux with stirring under a slow and continuous flow of nitrogen. The progress of the reaction was monitored at regular 30 min. intervals of time by taking the sample of the reaction mixture with a syringe. The initial brown color of the reaction mixture faded as the reaction progresses. After the completion of the reaction as indicated by TLC, the greenish reaction mixture was cooled and filtered through a celite pad. The insoluble green solid on the celite pad was washed thoroughly with chloroform and the light brown filtrate was evaporated under reduced pressure on a rotary evaporator. The residual mass was subjected to column chromatography using silica gel as the solid support and n-hexane and its mixture with ethyl acetate as the solvent for elution to get pure products in 45-70 % yield. The products were characterized by spectroscopic analysis, melting point and by comparing with authentic values.
References:
Ram, Ram N.;Tittal, Ram K. [Tetrahedron Letters,2016,vol. 57,# 22,p. 2437 - 2440] Location in patent:supporting information
2039-82-9
265 suppliers
$10.00/1g

4209-02-3
77 suppliers
$7.00/1g

99-90-1
468 suppliers
$6.00/25g

4209-02-3
77 suppliers
$7.00/1g
51556-09-3
0 suppliers
inquiry

4209-02-3
77 suppliers
$7.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)

