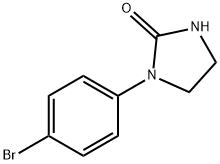
1-(4-Bromophenyl)imidazolidin-2-one synthesis
- Product Name:1-(4-Bromophenyl)imidazolidin-2-one
- CAS Number:530081-14-2
- Molecular formula:C9H9BrN2O
- Molecular Weight:241.08
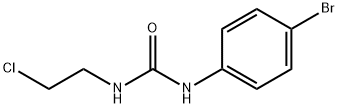
15145-38-7
23 suppliers
$137.00/1g

530081-14-2
28 suppliers
$43.00/100mg
Yield: 72%
Reaction Conditions:
with sodium hydride in tetrahydrofuran at 20; for 16.5 h;Cooling with ice;
Steps:
116B Example 1 16B: 1-(4-Bromophenyl)imidazolidin-2-one
To an ice cold mixture of Example 1 16A (8.00 g, 28.8 mmol) in THF (100 mL), was added sodium hydride (2.08 g, 860 mmol) in portions over 30 mm. The mixture was stirred at rt for 16 h, then was quenched with ice and diluted in ethyl acetate. The organiclayer was separated and washed with water and brine, dried (Na2SO4) and concentrated.This crude product was purified by triturating with petroleum ether to afford Example1 16B (5.00 g, 72% yield) as an off-white solid. MS(ESI) m/z: 241.3 (M+H) ‘H NMR(300MHz, DMSO-d6) ö 7.61 -7.35 (m, 1H), 7.05 (s, 1H), 3.82 (dd,J9.1, 6.8 Hz, 2H),3.44 - 3.35 (m, 2H).
References:
BRISTOL-MYERS SQUIBB COMPANY;GLUNZ, Peter W.;SITKOFF, Doree F.;YADAV, Navnath Dnyanoba;BODAS, Mandar Shrikrishna;BHIDE, Rajeev S.;PATIL, Sharanabasappa;CHINNAKOTTI, Kumaresan;RAO, Prasanna Savanor Maddu;SHETTY, Jeevanprakash WO2016/112236, 2016, A1 Location in patent:Page/Page column 192

120-93-4
536 suppliers
$10.00/5g

589-87-7
531 suppliers
$10.00/1g

530081-14-2
28 suppliers
$43.00/100mg

106-40-1
458 suppliers
$12.00/5g

530081-14-2
28 suppliers
$43.00/100mg