
1-(4-chloro-2-iodophenyl)-1H-Tetrazole synthesis
- Product Name:1-(4-chloro-2-iodophenyl)-1H-Tetrazole
- CAS Number:942316-74-7
- Molecular formula:C7H4ClIN4
- Molecular Weight:306.49
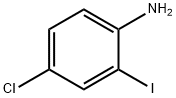
63069-48-7
231 suppliers
$15.00/10g
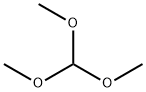
149-73-5
523 suppliers
$12.00/5g

942316-74-7
6 suppliers
inquiry
Yield:942316-74-7 92%
Reaction Conditions:
with sodium azide;acetic acid at 0 - 20; for 22.5 h;
Steps:
1.1E
Alternatively, IE can be prepared as follows. To a cold suspension(0-5 0C) of 4-chloro-2-iodoaniline (10.0 g, 39.5 mmol) and sodium azide (7.95 g, 122 mmol) in trimethyl orthoformate (13.08 mL, 118 mmol) was added acetic acid (150 mL). The resulting clear, slightly brown solution was stirred vigorously at 0-5 0C for 30 min and then warmed to rt. A beige precipitate formed overtime and then redissolved to give a clear brown solution. After 22 h, water (400 mL) was added and the suspension was stirred vigorously for 1 h. The solid was collected by filtration, rinsed with water, air-dried, and dried under vacuum to give 11.16 g (92%) of l-(4- chloro-2-iodo-phenyl)-lH-tetrazole as a beige solid. LCMS m/z, 307.0. (M+η)+. A flame-dried sealed tube vessel containing this intermediate (0.250 g, 0.816 mmol) and palladium acetate (0.018 g, 0.082 mmol) was purged with argon for several minutes. Next degassed acetonitrile (3.26 mL) was added followed by the addition of ethyl acrylate (0.133 mL, 1.224 mmol) and triethylamine (0.171 mL, 1.224 mmol). The vessel was sealed and the orange brown solution was warmed to 85 0C to give a brown suspension. After 21 h, the reaction was stopped and cooled to rt. The reaction was filtered through a 0.45 micron Glass microfibre (GMF), rinsing with acetonitrile, and the filtrate was concentrated to give a brown residue. Flash chromatography gave 0.098 g (43%) of (E)-3-(5-chloro-2-tetrazol-l-yl-phenyl)- acrylic acid ethyl ester as a pale yellow solid. LCMS m/z 279.1 (M+H)+ and 281 (M+2+H)+. Saponification as described above gave IE.
References:
WO2008/76805,2008,A2 Location in patent:Page/Page column 119