
[1-(4-chlorophenyl)cyclobutyl]methanamine synthesis
- Product Name:[1-(4-chlorophenyl)cyclobutyl]methanamine
- CAS Number:63010-09-3
- Molecular formula:C11H14ClN
- Molecular Weight:195.69
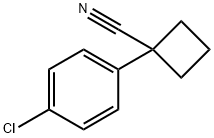
28049-61-8
168 suppliers
$15.00/250mg
![[1-(4-chlorophenyl)cyclobutyl]methanamine](/CAS/GIF/63010-09-3.gif)
63010-09-3
41 suppliers
$46.38/0.25g
Yield:63010-09-3 71%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20;Inert atmosphere;
Steps:
106.2 Step 2: Synthesis of 1[-1-(4-chlorophenyl)cyclobutyl]methanamine
In a round-bottomed flask, to a stirred solution of (4- 1- chlorophenyl)cyclobutanecarbonitrile (985 mg, 5.14 mmol) in anhydrous THF (22 mL) at 0 °Cunder nitrogenwas added 2.4 M LAH in THF (5.4 mL, 12.8 mmol). The reaction mixture was stirred at room temperature for 2 h. The reaction mixture was quenched at 0 °C with water (490 μL, same weight as LAH used). Next, 1 M sodium hydroxide (516 μL, 0.516 mmol) and water (three times the weight of LAH used) were added. The resulting heterogenous mixture was stirred at room temperature for 15 min, dried over sodium sulfate, filtered, rinsed with THF, and concentrated under reduced pressure. The crude was purified by flash chromatography on silica gel using a gradient of ammoniacal methanol in di chloromethane from 3% to 10% to afford the title compound as a yellow oil (715 mg, 71% yield, tr = 0.84 min). LCMS (Method F): m/z found 196.2 [M+H]+;1H-NMR (DMSO-d6, 400 MHz) δ (ppm) 7.40 - 7.29 (m, 2H), 7.16 - 7.04 (m, 2H), 2.75 (s, 2H), 2.20 - 2.08 (m, 4H), 2.05 - 1.91 (m, 1H), 1.83 - 1.71 (m, 1H), 1.11 (s, 2H).
References:
WO2022/167866,2022,A1 Location in patent:Page/Page column 256-257
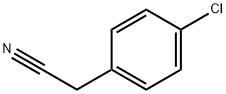
140-53-4
479 suppliers
$13.00/25g
![[1-(4-chlorophenyl)cyclobutyl]methanamine](/CAS/GIF/63010-09-3.gif)
63010-09-3
41 suppliers
$46.38/0.25g

28049-61-8
168 suppliers
$15.00/250mg

140-53-4
479 suppliers
$13.00/25g
![[1-(4-chlorophenyl)cyclobutyl]methanamine](/CAS/GIF/63010-09-3.gif)
63010-09-3
41 suppliers
$46.38/0.25g