
1-(4-fluoro-3-nitrobenzyl)piperidine synthesis
- Product Name:1-(4-fluoro-3-nitrobenzyl)piperidine
- CAS Number:509093-74-7
- Molecular formula:C12H15FN2O2
- Molecular Weight:238.26

110-89-4
3 suppliers
$15.96/100ML
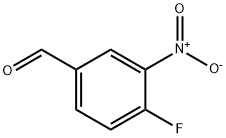
42564-51-2
209 suppliers
$5.00/1g

509093-74-7
15 suppliers
inquiry
Yield:-
Reaction Conditions:
with NaBH(OAc)3;acetic acid in 1,2-dichloro-ethane;
Steps:
29.c Example 29
(c) 1-(4-fluoro-3-nitrobenzyl)-piperidine To a 1 L flask containing 24.0 g 4-fluoro-3-nitrobenzaldehyde (142.0 mmol, 1.0 equiv), 400 mL 1,2-dichloroethane and 30 mL AcOH (426.0 mmol, 3.0 equiv) was carefully added a solution of 16.8 mL piperidine (170.4 mmol, 1.2 equiv) in 100 mL 1,2-dichloroethane via a addition funnel over a period of 1 h at 0° C., and then NaBH(OAc)3 (586.0 mmol, 4.0 equiv). After stirring for 12 h, the reaction was acidified (1 N HCl) and washed (2*hexane). The aqueous layer was then basified (solid NaOH), extracted (2*10% MeOH/CH2Cl2), dried (Na2SO4) and concentrated under reduced pressure to give 19.36 g of the product as a yellow liquid (81.3 mmol).
References:
US2003/144286,2003,A1
20274-69-5
112 suppliers
$10.00/250mg

509093-74-7
15 suppliers
inquiry
453-71-4
326 suppliers
$5.00/1g

509093-74-7
15 suppliers
inquiry