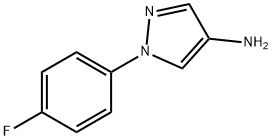
1-(4-fluorophenyl)-1H-pyrazol-4-amine synthesis
- Product Name:1-(4-fluorophenyl)-1H-pyrazol-4-amine
- CAS Number:1156602-69-5
- Molecular formula:C9H8FN3
- Molecular Weight:177.18
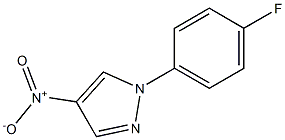
924709-31-9
3 suppliers
inquiry

1156602-69-5
11 suppliers
inquiry
Yield:1156602-69-5 63%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethanol;ethyl acetate; under 2068.65 Torr; for 1 h;
Steps:
5.b b)
Example 5 Synthesis of 3-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-l-yl]-l-[l-(4- fluorophenyl)pyrazol-4-yl]pyrrolidin-2-one [0108] a) A solution of 4-fluorophenylboronic acid (5.02 g, 35.4 mmol), 4-nitro-lH- pyrazole (2.00 g, 17.7 mmol), copper acetate (3.50 g, 19.5 mmol), and pyridine (7.00 mL, 88.5 mmol) in CH2CI2 (100 mL) was allowed to stir under air at room temperature for 12 h, The mixture was then filtered through a pad of Celite and the filtrate was concentrated in vacuo. The crude residue was purified by flash chromatography (S1O2, 30% EtOAc/hexanes) to afford the product as a white solid. [0109] b) A heavy-walled glass flask containing l-(4-fluorophenyl)-4-nitro-pyrazole (assumed 17.7 mmol) from step a and 10% Pd/C (0.30 g) in EtOH (50 mL) and EtOAc (10 mL) was fitted onto a Parr apparatus and agitated under at 40 psi. After 1 h, the reaction mixture was filtered through a pad of Celite and the filtrate was concentrated in vacuo to afford the product as a red solid (4.0 g, 22.6 mmol, 63% over two steps). The product was used without further purification. [0110] c) To a solution of 2-[4-chloro-5-methyl-3-(trifluoromethyl)pyrazol-l-yl]-4- hydroxy -butanoic acid (0.77 g, 2.7 mmol) and N,N-diisopropylethylamine (1.9 mL, 1 1 mmol) in CH2CI2 (30 mL) was added trimethylsilylchloride (0.85 mL, 6.8 mmol) at room temperature. The mixture was allowed to stir for 5 min before the addition of methanesulfonyl chloride (0.52 mL, 6.8 mL). After stirring for an additional 10 min, sodium bicarbonate (0.45 g, 5.4 mmol) and l-(4-fluorophenyl)pyrazol-4-amine (0.32 g, 1.8 mmol) from step a were each added as solids. The mixture was left to stir at room temperature for 12 h. The reaction was quenched by the addition of 1 Ν HC1 (30 mL) and extracted with CH2CI2 (1 x 50 mL). The organic layers were combined, dried over MgS04, filtered, and concentrated in vacuo. Purification of the crude material by flash chromatography (S1O2, 20%-50% EtOAc/hexanes) afforded the product as an orange oil (140 mg, 0.31 mmol, 18%). [0111] d) Diethyl azodicarboxylate (75 μ, 0.47 mmol) was slowly added to a solution of triphenylphosphine (124 mg, 0.47 mmol) in tetrahydrofuran (2 mL) under nitrogen. The yellow solution was allowed to stir for 20 min at room temperature before the alcohol intermediate (140 mg, 0.30 mmol) from step c was added as a solution in tetrahydrofuran (3 mL). After stirring overnight at room temperature, the reaction was quenched by the addition of saturated aqueous aHC03 and the mixture was extracted with CH2CI2 (2 x 20 mL). The organic layers were combined, dried over MgSC , filtered, and concentrated in vacuo. The crude material was purified by flash chromatography (Si02, 20-50% EtOAc/hexanes) and reverse phase HPLC (CI 8 column, acetonitrile-ELO with 0.1% TFA as eluent) to afford the titled compound (10 mg, 0.023 mmol, 8%) as a white solid. XH NMR (400 MHz, CDC13) δ 8.46 (s, 1 H), 7.74 (s, 1 H), 7.64 (dd, J= 9.2, 4.8 Hz, 2 H), 7.15 (dd, J= 8.8, 8.0 Hz, 2 H), 5.12 (dd, J= 9.3, 7.5 Hz, 1 H), 4.12 (ddd, J= 9.2, 9.2, 3.9 Hz, 1 H), 3.97-3.86 (m, 1 H), 3.10 (dddd, J= 14.0, 8.8, 6.8, 0.4 Hz, 1 H), 2.77 (dddd, J= 13.2, 9.6, 7.6, 3.6 Hz, 1 H), 2.44 (s, 3 H); MS: (ES) m/z calculated for CisH^C^ sO [M + H]+ 428.1, found 428.1.
References:
WO2014/89495,2014,A1 Location in patent:Paragraph 0107; 0109

957062-56-5
39 suppliers
$30.00/250mg

1156602-69-5
11 suppliers
inquiry

352-34-1
370 suppliers
$7.00/5g

1156602-69-5
11 suppliers
inquiry
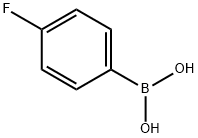
1765-93-1
560 suppliers
$9.00/1g

1156602-69-5
11 suppliers
inquiry