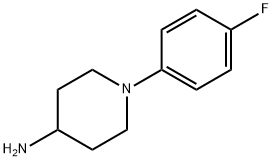
1-(4-fluorophenyl)piperidin-4-amine synthesis
- Product Name:1-(4-fluorophenyl)piperidin-4-amine
- CAS Number:164721-12-4
- Molecular formula:C11H15FN2
- Molecular Weight:194.25
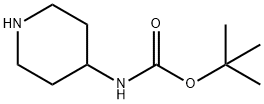
![Carbamic acid, N-[1-(4-fluorophenyl)-4-piperidinyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/483366-29-6.gif)
483366-29-6
0 suppliers
inquiry

164721-12-4
22 suppliers
$604.00/1g
Yield:164721-12-4 49%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane;water at 20; for 4 h;
Steps:
51.2 Step 2. Synthesis of l-(4-fluorophenyl)piperidin-4-amine
To a solution of the product from the previous step (590 mg, 2.00 mmol) in 1,4- dioxane (10 mL) was added 4M HCl/dioxane (4 mL) drop wise with stirring at rt over 2 min. The resulting solution was stirred for 4 h at rt. The pH was adjusted to 7 with 2 N NaHCCb. The resulting solution was extracted with 2x50 mL of EtOAc, and the combined organic layers were concentrated under vacuum to afford 190 mg (49%) of the title compound as an off-white solid. LC-MS: (ES, m/z) 195 lH NMR (400 MHz, DMSO -d6) d 7.10 - 6.97 (m, 2H), 6.97 - 6.90 (m, 2H), 3.55 - 3.44 (m, 2H), 2.65 (td, J = 11.9, 2.7 Hz, 3H), 1.76 (d, J = 12.7 Hz, 2H), 1.39 - 1.22 (m, 2H).
References:
WO2019/140188,2019,A1 Location in patent:Paragraph 0682; 0683; 0684
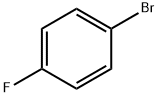
73874-95-0
449 suppliers
$10.00/250mg

164721-12-4
22 suppliers
$604.00/1g
460-00-4
608 suppliers
$6.00/10g

164721-12-4
22 suppliers
$604.00/1g