
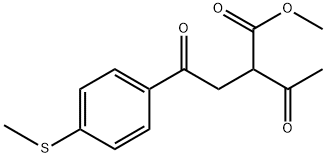
1-[4-(METHYLSULFANYL)PHENYL]-1,4-PENTANEDIONE synthesis
- Product Name:1-[4-(METHYLSULFANYL)PHENYL]-1,4-PENTANEDIONE
- CAS Number:189501-33-5
- Molecular formula:C12H14O2S
- Molecular Weight:222.3
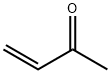
78-94-4
4 suppliers
$29.69/25ml
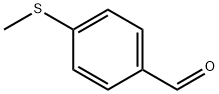
3446-89-7
207 suppliers
$14.00/1g
![1-[4-(METHYLSULFANYL)PHENYL]-1,4-PENTANEDIONE](/StructureFile/ChemBookStructure9/GIF/CB3414831.gif)
189501-33-5
27 suppliers
$181.00/500mg
Yield:189501-33-5 80%
Reaction Conditions:
with 3-ethyl-5-(2-hydroxyethyl)-4-methyl-1,3-thiazolium bromide;triethylamine at 70; under 7757.43 Torr; for 0.25 h;Microwave irradiation;Stetter 1,4-Dicarbonyl Synthesis;
Steps:
Synthesis of 1-[4-(methylthio)phenyl]pentane-1,4-dione (5).
A solution of 4-methylthiobenzaldehyde 4 (11.97 mL, 0.09 mol), triethylamine (19.5 mL, 0.14 mol), methyl vinyl ketone (5.8 mL, 0.09 mol), and 3-ethyl-5-(2-hydroxyethyl)-4-methylthiazolium bromide (3.53 g, 0.014 mol), in a 20 mL vial, was microwave irradiated using a CEM apparatus for 15 min at 70 °C (150 W, internal pressure of 150 psi). The reaction mixture was treated with 2 N HCl (10 mL) and extracted with ethyl acetate; the organic layer was washed with aqueous sodium bicarbonate and brine. The organic fractions were dried over sodium sulfate, filtered, and concentrated to give an orange liquid which was crystallized from cyclohexane to give intermediate 5 as white needles (80% yield). ESI-mass: m/z 245.063 [M+Na]+, mp, and 1H NMR spectrum were consistent with those reported in the literature. 16 and 23
References:
Battilocchio, Claudio;Poce, Giovanna;Alfonso, Salvatore;Porretta, Giulio Cesare;Consalvi, Sara;Sautebin, Lidia;Pace, Simona;Rossi, Antonietta;Ghelardini, Carla;Di Cesare Mannelli, Lorenzo;Schenone, Silvia;Giordani, Antonio;Di Francesco, Luigia;Patrignani, Paola;Biava, Mariangela [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 13,p. 3695 - 3701]
197905-69-4
1 suppliers
inquiry
![1-[4-(METHYLSULFANYL)PHENYL]-1,4-PENTANEDIONE](/StructureFile/ChemBookStructure9/GIF/CB3414831.gif)
189501-33-5
27 suppliers
$181.00/500mg

42445-46-5
39 suppliers
$62.50/250 mg
![1-[4-(METHYLSULFANYL)PHENYL]-1,4-PENTANEDIONE](/StructureFile/ChemBookStructure9/GIF/CB3414831.gif)
189501-33-5
27 suppliers
$181.00/500mg