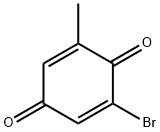
1,4-Naphthalenedione, 6-hydroxy-2-methyl- synthesis
- Product Name:1,4-Naphthalenedione, 6-hydroxy-2-methyl-
- CAS Number:633-71-6
- Molecular formula:C11H8O3
- Molecular Weight:188.18
Yield:633-71-6 70%
Reaction Conditions:
Stage #1: 2-bromo-6-methyl-[1,4]benzoquinone;1-methoxy-3-<(trimethylsilyl)oxy>01,3-butadiene in dichloromethane at 20; for 2 h;
Stage #2: with pyridine in dichloromethane;
Steps:
10.10.1
EXAMPLE 10: General procedure for Diels-Alder reaction with Danishefsky's diene: l-Methoxy-3-(trimethylsiloxy)-l,3-butadiene (2.0eq) was added dropwise to a methylbromoquinone (1.0 eq) in CH2C12 (0.2M). The solution was stirred at room temperature for 2 h, then pyridine (1.5eq) and Silica (ca. 1.5g/mmol) were added and the suspension stirred under air at rt for 6h. Concentration and flash column chromatography eluting with ethyl acetate/toluene (1 :2) gave the hydroxy-2-methylnaphthalene-l,4-dione.10.1 6-hydroxy-2-methylnaphthalene-l,4-dioneYield : 70% (Orange solid).m.p. 175 °C (from hexane/ethyl acetate).1H MR (300 MHz, CD3OCD3): δ 7.95 (d, J= 8.4 Hz, 1H), 7.39 (d, J= 2.5 Hz, 1H), 7.22 (dd, J= 8.4, 2.5 Hz, 1H), 6.82 (d, J= 1.6 Hz, 1H), 2.13 (d, J= 1.6 Hz, 3H) ppm.13C NMR (75 MHz, CD3OCD3) δ =185.5 (C=0), 184.5 (C=0), 163.4 (Cq), 149.4 (Cq), 135.9 (CH), 135.6 (Cq), 130.0 (CH), 125.9 (Cq), 121.3 (CH), 112.3 (CH), 16.4 (CH3) ppm.MS (EI) m/z (%): 188 ([M]+, 100), 160 (23).elemental analysis calcd (%) for CnH803 : C, 70.21; H, 4.29; found C 69.99, H 4.32The spectroscopic and physical data were identical to those reported in the literature (Bringmann G. and Al, 2011, 46, 5778-5789)
References:
WO2012/131010,2012,A1 Location in patent:Page/Page column 87-88
609-22-3
60 suppliers
$8.00/500mg

633-71-6
0 suppliers
inquiry
1345037-48-0
0 suppliers
inquiry

633-71-6
0 suppliers
inquiry

17579-79-2
48 suppliers
$91.00/100mg

633-71-6
0 suppliers
inquiry