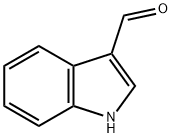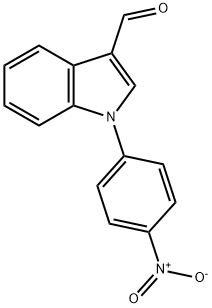
1-(4-nitrophenyl)-1H-indole-3-carbaldehyde synthesis
- Product Name:1-(4-nitrophenyl)-1H-indole-3-carbaldehyde
- CAS Number:65439-88-5
- Molecular formula:C15H10N2O3
- Molecular Weight:266.25
Yield:65439-88-5 80%
Reaction Conditions:
with copper;caesium carbonate;methyl-alpha-D-glucopyranoside in water;dimethyl sulfoxide at 100;Sealed tube;Green chemistry;
Steps:
General Procedure
General procedure: All the reactions were carried out in DMSO-H 2 O (1:1, 2 mL) in asealed vessel. To a 10 mL hydrothermal synthesis reactor was chargedCu powder (6 mg, 0.1 mmol), MG (39 mg, 0.2 mmol), Cs 2 CO 3 (980 mg,3.0 mmol), nitrogen-containing heterocycle (1.5 mmol), amine (3.0mmol), and aryl halide (0.8 mmol). The reaction mixture was stirredfor a specified time at 100-110 °C. After TLC analysis confirmed thecomplete consumption of aryl halides, the mixture was cooled to r.t.(the pH was adjusted if the product was acidic), diluted with EtOAc(10 mL), filtered through a Celite pad, and washed with EtOAc (20-30mL). The organic layer was dried and concentrated. The residue waspurified by silica gel column chromatography to give the product.
References:
Chen, Fengyang;Chen, Guoliang;Chen, Yuanguang;Du, Fangyu;Zhou, Qifan [Synthesis,2019,vol. 51,# 24,p. 4590 - 4600]