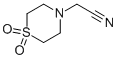
1-(4-SULFONO MORPHOLINE)ACETONITRILE synthesis
- Product Name:1-(4-SULFONO MORPHOLINE)ACETONITRILE
- CAS Number:79207-43-5
- Molecular formula:C6H10N2O2S
- Molecular Weight:174.22
39093-93-1
233 suppliers
$5.00/250mg

590-17-0
327 suppliers
$5.00/5g

79207-43-5
15 suppliers
inquiry
Yield:79207-43-5 91%
Reaction Conditions:
with potassium carbonate in acetonitrile at 60; for 16 h;
Steps:
98A.1
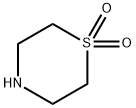
Step 1: (1,1-Dioxidothiomorpholin-4-yl)acetonitrile; 2.66 g (19.3 mmol) of potassium carbonate were added to a solution of 1.74 g (12.8 mmol) of thiomorpholine 1,1-dioxide [E. S. Lazer et al., J. Med. Chem. 2007, 37 (7), 913-923] and 1.69 g (14.1 mmol) of bromoacetonitrile in 30 ml of acetonitrile, and the mixture was stirred for 16 h at 60° C. After cooling, precipitated salts were filtered off and the filtrate was evaporated to dryness on a rotary evaporator. The residue obtained was purified by MPLC (silica gel, mobile phase: cyclohexane/ethyl acetate 1:1). 2.03 g (91% of theory) of the title compound were obtained.1H-NMR (400 MHz, CDCl3, δ/ppm): 3.61 (s, 2H), 3.13 (s, 8H).MS (DCI, NH3): m/z=192 [M+NH4]+.GC/MS (method I, EIpos): Rt=6.66 min, m/z=174 [M]+.
References:
US2011/312930,2011,A1 Location in patent:Page/Page column 65

39093-93-1
233 suppliers
$5.00/250mg

107-14-2
281 suppliers
$15.00/25g

79207-43-5
15 suppliers
inquiry