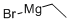
1-[4-(TRIFLUOROMETHOXY)PHENYL]CYCLOPROPAN-1-AMINE synthesis
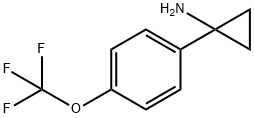
- Product Name:1-[4-(TRIFLUOROMETHOXY)PHENYL]CYCLOPROPAN-1-AMINE
- CAS Number:1200798-12-4
- Molecular formula:C10H10F3NO
- Molecular Weight:217.19
Yield:1200798-12-4 63%
Reaction Conditions:
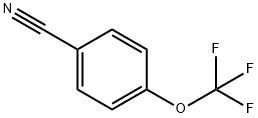
Stage #1: 4-(trifluoromethoxy)benzonitrile;ethylmagnesium bromidewith titanium(IV) isopropylate in diethyl ether at -70 - 20; for 1.83333 h;
Stage #2: with boron trifluoride diethyl etherate in diethyl ether at 20; for 3 h;
Steps:
110.a
Ethylmagnesium bromide (3.0 M in diethyl ether, 7.33 ml, 22.0 mmol) was added over 50 minutes to a solution of 4-(trifluoromethoxy)benzonitrile (1.87 g, 10.0 mmol) and tetraisopropoxytitanium (3.22 ml, 11.0 mmol) in diethyl ether (50 ml) at -70°C, and the whole was stirred at room temperature for 1 hour. Boranetrifluoride diethyl ether complex (2.53 ml, 20.0 mmol) was added over 15 minutes to the reaction mixture and the whole was stirred at room temperature for 3 hours. IN aqueous HCl solution (30 ml) and diethyl ether (90 ml) were added to the reaction mixture, and the whole was poured into aqueous 10 % NaOH solution (100 ml), extracted with diethyl ether (150 ml x 2), dried over Na2SO4, filtered and concentrated in vacuo. The residue was purified by column chromatography (ethyl acetate/hexane: 55/45 to 75/25) to give l-(4- (trifluoromethoxy)phenyl)cyclopropanamine (1.38 g, 63 %)
References:
WO2009/151152,2009,A1 Location in patent:Page/Page column 108