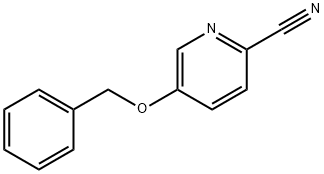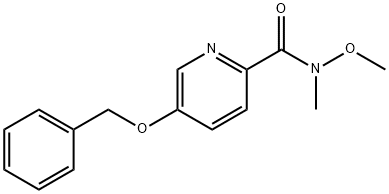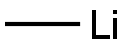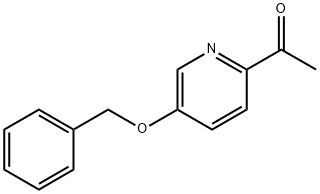
1-[5-(BENZYLOXY)PYRIDIN-2-YL]ETHANONE synthesis
- Product Name:1-[5-(BENZYLOXY)PYRIDIN-2-YL]ETHANONE
- CAS Number:858597-92-9
- Molecular formula:C14H13NO2
- Molecular Weight:227.26
Yield:858597-92-9 90%
Reaction Conditions:
in tetrahydrofuran; for 3 h;Inert atmosphere;Reflux;
Steps:
3 Example 3:
To a reflux condenser 20mL round bottom flask was added 5mL anhydrous tetrahydrofuran solution, 0.5mmol 5-benzyloxy-2-cyanopyridine,The mixture was stirred and replaced with nitrogen for three times. 1.5 mmol of methylmagnesium bromide was slowly added under the protection of nitrogen at room temperature. The whole system was heated to reflux and reacted for 3 hours.The whole reaction was poured into ice-water and extracted three times with dichloromethane, each time 20 mL, concentrated to give a white solid product, 102 mg, yield 90%.
References:
CN106397311,2017,A Location in patent:Paragraph 0012-0018

67310-56-9
78 suppliers
$47.00/100mg

100-39-0
425 suppliers
$10.00/10g
![1-[5-(BENZYLOXY)PYRIDIN-2-YL]ETHANONE](/CAS/GIF/858597-92-9.gif)
858597-92-9
10 suppliers
inquiry
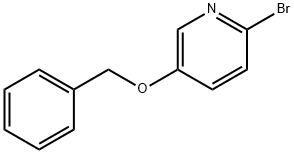
630120-99-9
52 suppliers
$32.00/250mg
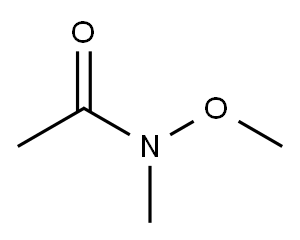
78191-00-1
268 suppliers
$6.00/5g
![1-[5-(BENZYLOXY)PYRIDIN-2-YL]ETHANONE](/CAS/GIF/858597-92-9.gif)
858597-92-9
10 suppliers
inquiry