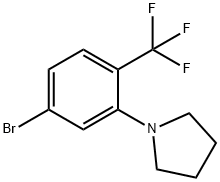
1-(5-Bromo-2-(trifluoromethyl)phenyl)pyrrolidine synthesis
- Product Name:1-(5-Bromo-2-(trifluoromethyl)phenyl)pyrrolidine
- CAS Number:1396780-07-6
- Molecular formula:C11H11BrF3N
- Molecular Weight:294.11
123-75-1
535 suppliers
$10.00/5g
142808-15-9
226 suppliers
$6.00/1g

1396780-07-6
4 suppliers
inquiry
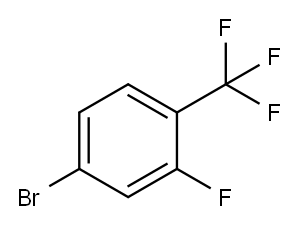
Yield:1396780-07-6 53%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in 1-methyl-pyrrolidin-2-one at 120;Sealed vessel;
Steps:
26
Example 26 Synthesis of TRV 1 158[00166] TRV 1158 - phenyl(4-(3-(pyrrolidin- l -yl)-4-(trifluoromethy])phenyl)piperazin- l - yl)methanone[00167] Scheme for TRV 1 158DIPEA / N P[00168] A mixture of 4-bromo-2-fluorobenzotrifluoride ( l .5 l l g, 6.2 mmol), pyrrolidine (0.62 mL, 7.46 mmol), DIPEA ( 1.6 mL, 9.33 mmol) and NMP (8 mL) were sealed in a tube and heated to 120 °C overnight. The solution was cooled to room temperature and diluted with water and EtOAc. The layers were separated and the aqueous layer was back-extracted with EtOAc (3x). The combined organic layers were then washed successively with H2O, IN HCl(aq), saturated NaHC03(aq), H2O, and brine before drying with Na2SC>4. The mixture was filtered and concentrated to give a crude oil, which was purified via flash chromatography (5 % EtOAc / hexane) to give 0.9667 g (53 % yield) of desired product. This bromo-intermediate (0.5312 g, 1.8 mmol), benzoylpiperazine hydrochloride (0.4987 g, 2.2 mmol) and NaO/Bu (0.5189 g, 5.4 mmol) were charged to a flask which was subsequently purged and evacuated with argon (3 cycles). Toluene (5.4 mL) and NMP (3.2 mL) were then added and the solution was degassed for 30 minutes before added Pd2(dba)3 (0.033 g, 0.036 mmol) and BINAP (0.045 g, 0.072 mmol) all at once. The flask was then heated to 100 °C overnight under argon. The mixture was cooled to room temperature and diluted with EtOAC before filtering through Celite. The organic layer was then washed successively with H20, IN HCl(aq), saturated NaHC03(aq), H2O, and brine before drying with Na2S04. The mixture was filtered and concentrated to give 0.5081 g of crude oil. The oil was purified via flash chromatography (45 % EtOAc / hexane) to give another crude oil that was slightly impure. This oil was crystallized from EtOAc (solvent) and hexane (anti-solvent) to give 0.101 g of white crystals of TRV 1158, phenyl(4-(3-(pyrrolidin-l -yl)-4- (trifluoromethyl)phenyl)piperazin- l-yl)methanone. NMR (500 MHz, DMSO) δ = 7.47-7.41 (m, 5H), 7.35 (d, J = 8.5 Hz, 1 H), 6.49 (dd, J = 8.5, 1.5 Hz, 1 H), 6.46 (m, 1 H), 3.72 (br s, 2H), 3.45 (br s, 2H), 3.30 (br s, 4H), 3.23-3.20 (m, 4H), 1.88- 1.83 (m, 4H).
References:
WO2012/119035,2012,A1 Location in patent:Page/Page column 58-59