
1-(5-bromo-3-pyridinyl)-1-propanone synthesis
- Product Name:1-(5-bromo-3-pyridinyl)-1-propanone
- CAS Number:341555-43-9
- Molecular formula:C8H8BrNO
- Molecular Weight:214.06

2386-64-3
215 suppliers
$45.00/5ml
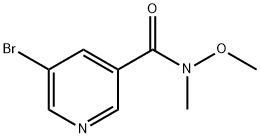
183608-47-1
43 suppliers
$110.00/500mg

341555-43-9
16 suppliers
inquiry
Yield:341555-43-9 88%
Reaction Conditions:
in tetrahydrofuran at 10; for 4 h;
Steps:
1.1 1. Synthesis of (5-bromopyridin-3-yl) ethyl ketone
20.8 g of N-methyl-N-methoxy-5-bromo-nicotinamide was dissolved in 200 ml of tetrahydrofuran, cooled to 10 degrees, and 45 ml of a 2 M solution of ethylmagnesium chloride in tetrahydrofuran was added.After the reaction was continued for 4 hours, it was warmed to room temperature and quenched with water.It was extracted twice with ethyl acetate and the organic phase was desolvated. Distillation under reduced pressure gave 16 g of a pale yellow solid with a yield of 88%.
References:
CN107954927,2018,A Location in patent:Paragraph 0016
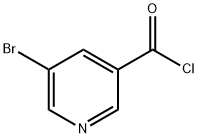
39620-02-5
75 suppliers
$82.00/10g
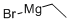
925-90-6
354 suppliers
$12.00/5ml

341555-43-9
16 suppliers
inquiry

811-49-4
59 suppliers
$140.00/4x25ml

183608-47-1
43 suppliers
$110.00/500mg

341555-43-9
16 suppliers
inquiry
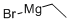
925-90-6
354 suppliers
$12.00/5ml

183608-47-1
43 suppliers
$110.00/500mg

341555-43-9
16 suppliers
inquiry
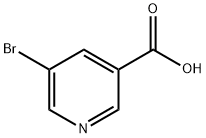
20826-04-4
554 suppliers
$5.00/1g

341555-43-9
16 suppliers
inquiry