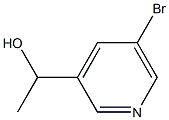
1-(5-bromopyridin-3-yl)ethanol synthesis
- Product Name:1-(5-bromopyridin-3-yl)ethanol
- CAS Number:283608-66-2
- Molecular formula:C7H8BrNO
- Molecular Weight:202.05
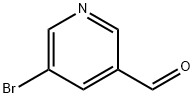
113118-81-3
279 suppliers
$5.00/250mg

75-16-1
265 suppliers
$12.00/10ml

283608-66-2
31 suppliers
$76.00/250mg
Yield:283608-66-2 98.1%
Reaction Conditions:
in tetrahydrofuran at 20; for 1 h;
Steps:
3.1 Step 1:
5-Bromonicotinic aldehyde (980mg, 5.0mmol), dissolved in 45mL THF, 1M methylmagnesium bromide solution (10mL, 10mmol) was added to the reaction at room temperature for 1 hour, 50mL saturated ammonium chloride was added to the reaction solution The aqueous solution was extracted with dichloromethane (100 mL×2), the organic phases were combined, washed with brine (100 mL×2), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure.Get 1-(5-bromopyridin-3-yl)ethanol(1.1 g, colorless liquid), yield: 98.1%
References:
CN111808086,2020,A Location in patent:Paragraph 0132-0134
38940-62-4
232 suppliers
$6.00/250mg

283608-66-2
31 suppliers
$76.00/250mg