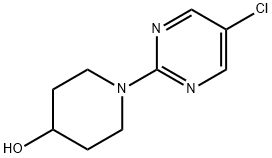
1-(5-Chloro-pyrimidin-2-yl)-piperidin-4-ol synthesis
- Product Name:1-(5-Chloro-pyrimidin-2-yl)-piperidin-4-ol
- CAS Number:1108164-37-9
- Molecular formula:C9H12ClN3O
- Molecular Weight:213.66
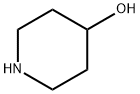
5382-16-1
13 suppliers
$9.00/5G
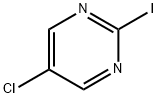
874676-81-0
121 suppliers
$10.00/250mg

1108164-37-9
5 suppliers
$685.00/1g
Yield:1108164-37-9 87%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 20; for 16 h;
Steps:
4
1-(5-Chloropyrimidin-2-yl)piperidin-4-ol To a one dram vial was added piperidin-4-ol (101 mg, 1 mmol), 5-chloro-2-iodopyrimidine (240 mg, 1.000 mmol), and THF (1 mL) to produce a suspension. To this mixture was added TEA (0.153 mL, 1.100 mmol). The reaction was stirred at room temperature for 16 hours. Ethyl acetate (7 mL) was added at that point and the mixture was washed with water (4*2 mL). The organic layer was dried with magnesium sulfate, filtered, and concentrated in vacuo. The residue was purified by flash chromatography (silica gel, 0-40% ethyl acetate/hexane) to give Intermediate 4 (185 mg, 87% yield) as white powder. 1H NMR (CDCl3, 500 MHz) δ 8.22 (2H, s), 4.24-4.44 (2H, m), 3.83-4.04 (1H, m), 3.15-3.50 (2H, m), 1.83-2.02 (2H, m), 1.40-1.60 (2H, m); MS (ESI) 214.2 (M+H).
References:
US2011/251221,2011,A1 Location in patent:Page/Page column 14

5382-16-1
13 suppliers
$9.00/5G
22536-67-0
270 suppliers
$10.00/5g

1108164-37-9
5 suppliers
$685.00/1g