
1,5-DIBENZYL GLUTARATE synthesis
- Product Name:1,5-DIBENZYL GLUTARATE
- CAS Number:56977-08-3
- Molecular formula:C19H20O4
- Molecular Weight:312.36
201230-82-2
1 suppliers
inquiry
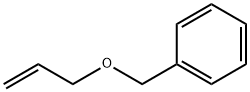
14593-43-2
121 suppliers
$18.00/1g
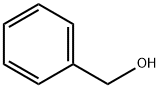
100-51-6
1384 suppliers
$5.00/100g

56977-08-3
18 suppliers
$115.00/500mg
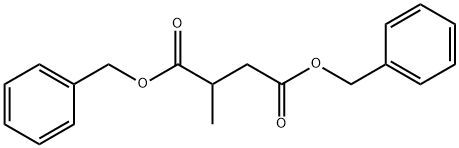
73773-32-7
0 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogenchloride;palladium diacetate;4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in diethyl ether;toluene at 110; under 30003 Torr; for 24 h;Inert atmosphere;Autoclave;Overall yield = 70 %Chromat.;
Steps:
8.4
A 4 ml glass vial is charged with [Pd(OAc)2] (2.24 mg, 1.0 mol %), Xantphos (L1) (8.7 mg, 1.5 mol %) and a magnetic stirrer. This vial is closed with a phenolic resin cap consisting of a septum made from Teflon-coated styrene-butadiene rubber. This septum is pierced with a cannula, and the atmosphere in the vial is replaced by an argon atmosphere through this cannula by three cycles of application of reduced pressure and purging with argon. Through this cannula, 2 ml of toluene, benzyl allyl ether (148 mg, 1.0 mmol), BnOH (162 mg, 1.5 mmol) and 1 M hydrochloric acid solution in diethyl ether (20 μl, 2 mol %) are then injected by means of syringes. This vial is then placed in a metal plate which is then transferred into a 300 ml steel autoclave from Parr Instruments under an argon atmosphere. After the autoclave has been purged three times with CO, CO is injected to 40 bar at room temperature. The reaction is conducted while stirring with a magnetic stirrer at 110° C. for 24 hours. After the end of the reaction, the autoclave is cooled down and the pressure is released gradually. The autoclave is purged three times with nitrogen. Subsequently, dodecane (100 μl) is added as internal standard. The yield and selectivity are determined by means of GC analysis.
References:
US2017/174609,2017,A1 Location in patent:Paragraph 0095; 0099
201230-82-2
1 suppliers
inquiry

107-18-6
2 suppliers
$24.60/100ml

100-51-6
1384 suppliers
$5.00/100g

56977-08-3
18 suppliers
$115.00/500mg

73773-32-7
0 suppliers
inquiry

108-55-4
431 suppliers
$6.00/25g

100-51-6
1384 suppliers
$5.00/100g

56977-08-3
18 suppliers
$115.00/500mg
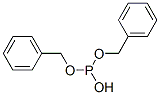
538-60-3
39 suppliers
$110.00/25g
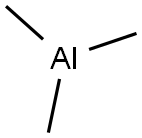
75-24-1
152 suppliers
$54.50/100ml

56977-08-3
18 suppliers
$115.00/500mg
![Pentanedioic acid, 2-[[bis(phenylmethoxy)phosphinyl]methyl]-, 1,5-bis(phenylmethyl) ester](/CAS/20210305/GIF/173039-06-0.gif)
173039-06-0
1 suppliers
inquiry

110-94-1
497 suppliers
$5.00/25g
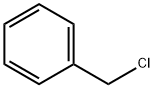
100-44-7
627 suppliers
$13.50/250G

56977-08-3
18 suppliers
$115.00/500mg
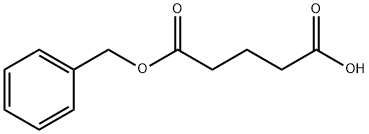
54322-10-0
48 suppliers
$16.00/250mg