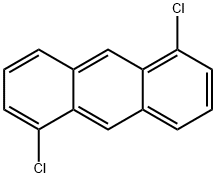
1,5-Dichloroanthracene synthesis
- Product Name:1,5-Dichloroanthracene
- CAS Number:6406-96-8
- Molecular formula:C14H8Cl2
- Molecular Weight:247.12
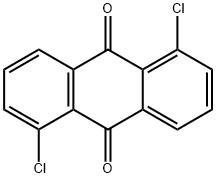
82-46-2
216 suppliers
$5.00/1g

6406-96-8
5 suppliers
inquiry
Yield:6406-96-8 84.45%
Reaction Conditions:
with ammonium hydroxide;zinc at 100; for 3 h;
Steps:
3.2.1. Synthesis of 1,5-Dichloroanthracene 3
1,5-Dichloroanthraquinone 4 (10.0 g, 36.1 mmol) and zincdust (50.0 g, 765 mmol) were suspended in 200mL ofaqueous 28% NH3 with stirring for 3 h at 100 degree Celsius.After cooling to r.t., the reaction mixture was separated byltration and the ltrate was eluted and extracted severaltimes with CH2Cl2. &e CH2Cl2 organic phases were collected,dried over MgSO4, ltered, and concentrated under avacuum. To the residual crude, 500mL isopropanol and50mL aqueous HC1 (12 M) were added. After reBuxing thereaction mixture for 3 h, the mixture was cooled, concentratedin vacuo and then partitioned between CH2Cl2 andaqueous 5% NaHC03. &e CH2Cl2 organic phase was collected,dried with MgSO4, ltered, and concentrated to givecrude 1,5-dichloroanthracene 3. &is crude was crystallizedusing a CH2Cl2-petroleum ether mixture. &e product 1,5-dichloroanthracene 3 was dried in the air for 24 h (7.5 g,30.49 mmol) as yellow-orange needles in a 84.45% yield; mp187-188 Celsius degree; IR (KBr): 9 681, 720, 736, 778, 849,873, 903, 958, 996, 1145, 1162, 1212, 1300, 1449, 1530, and1618 cm-1; 1H-NMR (CDCl3, 400 MHz): 3 7.40-7.61 (m, 4H, ArH), 8.00 (dd; J 2.92 Hz, 2 H, ArH), 8.42 (s, 1 H, ArH),8.84 (s, 1 H, ArH) ppm. 13C NMR (CDCl3, 100 MHz):3 124.37, 125.42, 125.58, 126.29, 128.24, 131.7, 132.8 ppm.
References:
Sultan, Mujeeb A.;Pillai, Renjith Raveendran;Alzahrani, Eman;Alsofi, Ahmed A.;Al-Qadhi, Sadam A.;Pashameah, Rami Adel [Journal of Chemistry,2022,vol. 2022,art. no. 1196244]
50259-92-2
0 suppliers
inquiry

6406-96-8
5 suppliers
inquiry
860729-36-8
0 suppliers
inquiry

6406-96-8
5 suppliers
inquiry
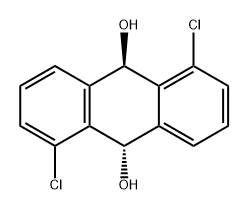
41187-73-9
0 suppliers
inquiry

6406-96-8
5 suppliers
inquiry
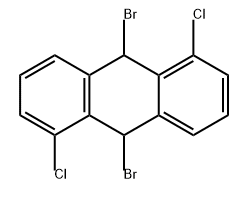
855952-61-3
0 suppliers
inquiry
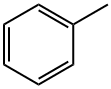
108-88-3
7 suppliers
$14.00/100ml

74-89-5
7 suppliers
$13.44/25ML

6406-96-8
5 suppliers
inquiry