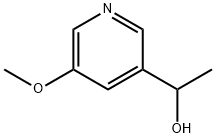
1-(5-METHOXYPYRIDIN-3-YL)ETHAN-1-OL synthesis
- Product Name:1-(5-METHOXYPYRIDIN-3-YL)ETHAN-1-OL
- CAS Number:35307-06-3
- Molecular formula:C8H11NO2
- Molecular Weight:153.18
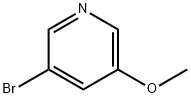
113118-83-5
143 suppliers
$13.00/250mg
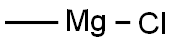
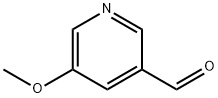
676-58-4
276 suppliers
$15.00/25ml

35307-06-3
5 suppliers
inquiry
Yield:35307-06-3 560 mg
Reaction Conditions:
Stage #1: 5-methoxypyridine-3-carboxaldehyde;methylmagnesium chloride in tetrahydrofuran at 0; for 1 h;
Stage #2: with ammonium chloride in tetrahydrofuran;water at 0; for 0.5 h;
Steps:
22-7-1.1
A 3.0 mol/L methyl magnesiumchloride tetrahydrofuran solution (2.6 mL) was added to a solution of 5-methoxynicotinic aldehyde (707 mg) in tetrahydrofuran (20 mL) at 0° C., followed by stirring for 1 hour. A saturated ammonium chloride aqueous solution (3 mL) was added to the reaction mixture, followed by stirring at 0° C. for 0.5 hours, and a saturated sodium hydrogen carbonate aqueous solution was added thereto. The resultant product was extracted with ethyl acetate, the organic layer was washed with a saturated sodium chloride aqueous solution and dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure, whereby 1-(5-methoxypyridin-3-yl)ethanol (560 mg) was obtained as an orange oily material. (1183) 1H-NMR (CDCl3, 300 MHz) δ:8.27-8.17 (2H, m), 7.32-7.24 (1H, m), 5.04-4.91 (1H, m), 3.92-3.85 (3H, m), 2.09-1.96 (1H, m), 1.58-1.50 (3H, m). (1184) MS(ESI m/z): 154 (M+H) (1185) RT(min): 0.28
References:
US2016/168139,2016,A1 Location in patent:Paragraph 1180-1185