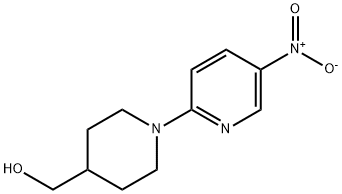
(1-(5-nitropyridin-2-yl)piperidin-4-yl)methanol synthesis
- Product Name:(1-(5-nitropyridin-2-yl)piperidin-4-yl)methanol
- CAS Number:1227935-27-4
- Molecular formula:C11H15N3O3
- Molecular Weight:237.26
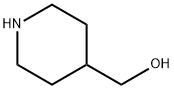
6457-49-4
386 suppliers
$18.00/25g
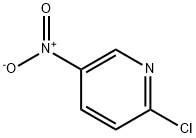
4548-45-2
536 suppliers
$6.00/5g

1227935-27-4
9 suppliers
inquiry
Yield:1227935-27-4 50.4%
Reaction Conditions:
in ethanol at 80; for 2 h;
Steps:
1 Step 1: Preparation of (1-(5-nitropyridin-2-yl)piperidin-4-yl)methanol
To a solution of 2-chloro-5-nitropyridine (5 g, 31.5 mmol) in EtOH (50 mL) stirred at room temperature was added piperidin-4-ylmethanol (3.63 g, 31.5 mmol). The reaction mixture was stirred at 80° C. for 2 hours. After the reaction completion, the mixture was cooled to room temperature and concentrated under vacuum. The residue was purified by flash column chromatography on silica gel (PE/EtOAc=3:1) to afford the desired product (3.77 g, 50.4%) as a yellow solid. LC/MS: 238.0 [M+H]+; 1H NMR (400 MHz, CDCl3) δ=9.05 (d, J=2.8 Hz, 1H), 8.20 (dd, J=9.6, 2.8 Hz, 1H), 6.60 (d, J=9.6 Hz, 1H), 4.59 (d, J=12.8 Hz, 2H), 3.57 (d, J=5.6 Hz, 2H), 3.05 (t, J=12.8 Hz, 2H), 1.97-1.90 (m, 2H), 1.90-1.84 (m, 1H), 1.37-1.24 (m, 2H).
References:
US2021/87170,2021,A1 Location in patent:Paragraph 0223-0224
1227862-56-7
0 suppliers
inquiry

1227935-27-4
9 suppliers
inquiry
40807-61-2
186 suppliers
$28.00/1g

1227935-27-4
9 suppliers
inquiry

4548-45-2
536 suppliers
$6.00/5g

1227935-27-4
9 suppliers
inquiry