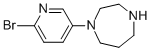
1-(6-Bromo-3-pyridyl)homopiperazine synthesis
- Product Name:1-(6-Bromo-3-pyridyl)homopiperazine
- CAS Number:223797-21-5
- Molecular formula:C10H14BrN3
- Molecular Weight:0
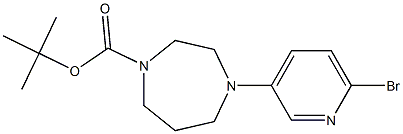
223797-58-8
4 suppliers
inquiry
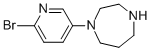
223797-21-5
8 suppliers
inquiry
Yield:223797-21-5 64%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 20; for 16 h;
Steps:
24 3.2.24. 1-(6-Bromopyridin-3-yl)-4-(3-phenylpropyl)-1,4-diazepane (28)
A solution of 58 (598 mg, 1.68 mmol) and TFA (2.58 mL, 33.7 mmol) in DCM (20 mL) was stirred at rt for 16 h.
The reaction mixture was evaporated in vacuo.
The residue was added aq NaOH solution (4 M, 20 mL) and extracted with DCM (3 * 100 mL).
The combined organic phases were dried (MgSO4), filtered, and evaporated in vacuo giving 1-(6-bromopyridin-3-yl)-1,4-diazepane as a yellow oil (274 mg, 64%) that crystallizes over time. 1H NMR (CDCl3, 400 MHz) δ 7.84 (d, 1H, J = 3.3 Hz), 7.23 (dd, 1H, J = 8.8, 0.5 Hz), 6.86 (dd, 1H, J = 8.9, 3.4 Hz), 3.54 (dt, 4H, J = 16.7, 5.8 Hz), 3.08-3.00 (m, 2H), 2.89-2.80 (m, 2H), 2.28 (bs, 1H), 1.97-1.85 (m, 2H).
13C NMR (CDCl3, 101 MHz) δ 143.90, 133.91, 127.59, 126.76, 121.08, 51.71, 47.95, 47.91, 47.89, 28.92.
References:
Bach, Tinna B.;Jensen, Anders A.;Petersen, Jette G.;S?rensen, Troels E.;Della Volpe, Serena;Liu, Jun;Blaazer, Antoni R.;Van Muijlwijk-Koezen, Jacqueline E.;Balle, Thomas;Fr?lund, Bente [European Journal of Medicinal Chemistry,2015,vol. 102,art. no. 8008,p. 425 - 444]

223796-20-1
36 suppliers
inquiry
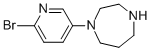
223797-21-5
8 suppliers
inquiry
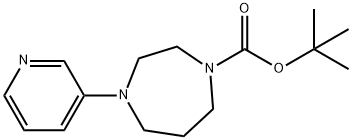
223797-48-6
2 suppliers
inquiry
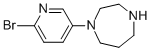
223797-21-5
8 suppliers
inquiry
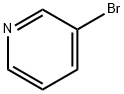
626-55-1
563 suppliers
$5.00/5/ G
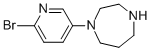
223797-21-5
8 suppliers
inquiry
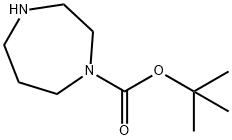
112275-50-0
249 suppliers
$9.00/1g
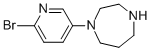
223797-21-5
8 suppliers
inquiry