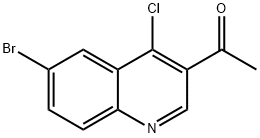
1-(6-BROMO-4-CHLOROQUINOLIN-3-YL)ETHANONE synthesis
- Product Name:1-(6-BROMO-4-CHLOROQUINOLIN-3-YL)ETHANONE
- CAS Number:1255099-21-8
- Molecular formula:C11H7BrClNO
- Molecular Weight:284.54

99867-16-0
8 suppliers
inquiry

1255099-21-8
12 suppliers
$45.00/10mg
Yield:1255099-21-8 72%
Reaction Conditions:
with trichlorophosphate at 85; for 3 h;
Steps:
395
Example 395l -(6-Bromo-4-chloroquinolin-3-yl)ethanonel -(6-Bromo-4-hydroxyquinolin-3-yl)ethanone (2.2 g, 8.27 mmol) was suspended in phosphoryl chloride (30 mL) and the reaction was heated to 85 °C and stirred for 3 h. After this time the reaction mixture was cooled to room temperature and slowly poured into a 2: 1 solution of satd. aq. sodium bicarbonate/ ethyl acetate that was cooled to 0 °C. The organic layer was separated, washed with satd. aq. sodium bicarbonate, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography to afford the desired product (710 mg, 72%) as a light brown solid: ESI MS m/z 280 [C| ,H7BrClNO + H]+.
References:
WO2012/16082,2012,A1 Location in patent:Page/Page column 262

106-40-1
457 suppliers
$12.00/5g

1255099-21-8
12 suppliers
$45.00/10mg
![Butanoic acid, 2-[[(4-bromophenyl)amino]methylene]-3-oxo-, ethyl ester](/CAS/20200515/GIF/100380-48-1.gif)
100380-48-1
0 suppliers
inquiry

1255099-21-8
12 suppliers
$45.00/10mg