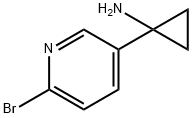
1-(6-BROMOPYRIDIN-3-YL)CYCLOPROPAN-1-AMINE synthesis
- Product Name:1-(6-BROMOPYRIDIN-3-YL)CYCLOPROPAN-1-AMINE
- CAS Number:1060811-36-0
- Molecular formula:C8H9BrN2
- Molecular Weight:213.07
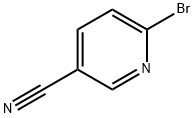
139585-70-9
303 suppliers
$10.00/1g

1060811-36-0
9 suppliers
inquiry
Yield:1060811-36-0 203 mg
Reaction Conditions:
Stage #1: 2-bromo-5-cyanopyridinewith titanium(IV) isopropylate;ethylmagnesium bromide in diethyl ether at 15 - 20; for 0.5 h;
Stage #2: with boron trifluoride diethyl etherate in diethyl ether at 20; for 0.75 h;
Steps:
II 1-(6-bromopyridin-3-yl)cyclopropan-1-amine
732 mg (4.00 mmol) 6-Bromopyridine-3-carbonitrile (CAS No. 139585-70-9) is diluted with 30 mL diethylether and 1.37 mL (4.67 mmol) titantetraisopropylate is added dropwise at RT. To the mixture is added 2.95 mL (8.84 mmol) ethylmagnesiumbromide (3 M in di ethylether) under cooling at 15 - 20 °C. The reaction mixture is stirred at RT for 30 min. Under cooling 1.26 mL (9.97mmol) borotrifluoride-diethyletherate is added to the mixture and it is stirred at RT for 45 min. The reaction mixture is quenched with 20 mL 2 N NaOH under cooling, stirred at RT for 2 h and filtered through celite. The filter cake is washed with diethylether. The water phase of the filtrate is extracted with diethylether and all of the organic layers are reduced in vacuo. The residue is purified by HPLC to afford 203 mg of the product. C8H9BrN2 (M = 436.3 g/mol) ESI-MS: 214/216 Br [M+H]+ Rt (HPLC): 0.92 min (method B)
References:
WO2021/13833,2021,A1 Location in patent:Page/Page column 28
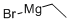
925-90-6
344 suppliers
$12.00/5ml

139585-70-9
303 suppliers
$10.00/1g

1060811-36-0
9 suppliers
inquiry

75-16-1
267 suppliers
$12.00/10ml

139585-70-9
303 suppliers
$10.00/1g

1060811-36-0
9 suppliers
inquiry