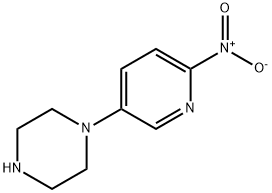
1-(6-nitropyridin-3-yl)piperazine synthesis
- Product Name:1-(6-nitropyridin-3-yl)piperazine
- CAS Number:775288-71-6
- Molecular formula:C9H12N4O2
- Molecular Weight:208.22
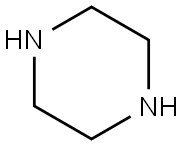
110-85-0
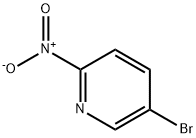
39856-50-3

775288-71-6
GENERAL METHODS: A mixture of 5-bromo-2-nitropyridine (24.7 mmol), piperazine (29.2 mmol) and N,N-diisopropylethylamine (DIPEA, 37.1 mmol) in acetonitrile (100 mL) was refluxed for 8 hours. The reaction progress was monitored by thin layer chromatography (TLC), and after complete conversion of the feedstock, the reaction mixture was cooled to room temperature. The solvent was removed by evaporation and the residue was purified by column chromatography to afford 1-(6-nitropyridin-3-yl)piperazine (8c). The product was a yellow solid in 82% yield.1H NMR (DMSO-d6, 400 MHz) δ: 7.96 (d, J = 9.3 Hz, 1H, Pyr-H), 7.88 (d, J = 2.9 Hz, 1H, Pyr-H), 7.26 (dd, J = 9.3, 3.0 Hz, 1H, Pyr-H), 3.64 (t, 4H, Pyr -NCH2CH2N), 3.32 (t, 4H, Pyr-NCH2CH2N).13C NMR (DMSO-d6, 100 MHz): δ 151.7, 146.2, 136.6, 125.9, 118.4, 51.3, 45.8. High-resolution mass spectrometry (HRMS, ESI): the calculated value for C9H12N4O2 [M + H]+is 209.1039 and the measured value is 209.1030.

110-85-0
590 suppliers
$5.00/5 g

39856-50-3
544 suppliers
$6.00/5g

775288-71-6
109 suppliers
inquiry
Yield: 94.8%
Reaction Conditions:
with modified hectorite-loaded ionic liquid in dimethyl sulfoxide at 80; for 5 h;Green chemistry;Solvent;Reagent/catalyst;
Steps:
4 Example 4
1) 5-Bromo-2-nitropyridine (1 mol, 203 g) was dissolved in 1.5 L of dimethyl sulfoxide, and then an organically modified hectorite-supported ionic liquid material MHIL (40.6 g, 20 wt%) was added. Agitating and dispersing uniformly to obtain a first mixed liquid;2) Piperazine (129.2 g, 1.5 mol) was dissolved in 800 ml of dimethyl sulfoxide to form a piperazine solution, and then the piperazine solution was added dropwise to the first mixture to carry out a condensation reaction at 80 ° C;3) After 5 hours, the reaction solution was taken for HPLC detection (the percentage of 5-bromo-2-nitropyridine in the reaction solution was 0.08%, and the target product 1-(6-nitropyridin-3-yl)piperazine was 99.68%. Double condensation by-product 0.17%, the balance is unknown impurities), stop the reaction, use an organic microporous membrane with a pore size of 0.5 μm to filter and remove the organic modified hectorite-loaded ionic liquid material to obtain a filtrate;4) The filtrate is warmed to 60-65 ° C, and then the aqueous solution of methylamine at a concentration of 3 V% is added dropwise. When the turbidity occurs in the system, the dropwise addition is stopped, the mixture is kept warm for 20-30 min, and then the aqueous solution of methylamine having a concentration of 3 V% is continuously added dropwise. The concentration of 1-(6-nitropyridin-3-yl)piperazine in the solution was no longer decreased, and the temperature was naturally lowered to room temperature, filtered, and dried under vacuum at 45 ° C to obtain 197.4 g of a solid. The yield was 94.8%. 99.92% (external standard method); LC-MS: m/z = 209.1 [M+H].The ionic liquid material supported by the organic modified hectorite filtered by filtration was air-dried by acetone and recovered, and the yield of 1-(6-nitropyridin-3-yl)piperazine was 94.2%, and the content was 99.89%. Compared with the catalytic effect of fresh preparation, the catalytic material prepared by the invention can be recycled and used to reduce the production cost of the condensation step.
References:
Zheng Chuanhua;Lu Xueling;Zhang Lei CN109369517, 2019, A Location in patent:Paragraph 0053; 0060; 0064-0069
571189-16-7
202 suppliers
$9.00/250mg

775288-71-6
109 suppliers
inquiry

110-85-0
590 suppliers
$5.00/5 g
52092-47-4
355 suppliers
$6.00/1g

775288-71-6
109 suppliers
inquiry

39856-50-3
544 suppliers
$6.00/5g

775288-71-6
109 suppliers
inquiry