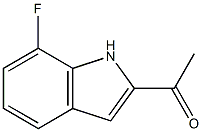
1-(7-Fluoro-1H-indol-2-yl)ethanone synthesis
- Product Name:1-(7-Fluoro-1H-indol-2-yl)ethanone
- CAS Number:1373438-98-2
- Molecular formula:C10H8FNO
- Molecular Weight:177.175

399-67-7
104 suppliers
$18.00/100mg
917-54-4
176 suppliers
$52.10/25ml

1373438-98-2
3 suppliers
inquiry
Yield:1373438-98-2 45%
Reaction Conditions:
in diethyl ether at 0; for 2 h;Reflux;
Steps:
General procedure for the synthesis of substituted 1-(1H-indol-2-yl)ethanone
General procedure: To a solution of the appropriate 1H-indole-2-carboxylic acid 1 (5 mmol) in Et2O (20 mL) at 0°C was added MeLi (10.9 mL, 1.6M in Et2O, 17.4 mmol) dropwise. The mixture reaction was refluxed for 2 h, cooled to room temperature and neutralized with 10% aqueous hydrochloric acid. The organic layer was separated and the aqueous layer was extracted with EtOAc (3?15 mL). The organic phases were combined, washed with brine, dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The crude product was purified by flash column chromatography using 80/20 hexanes/EtOAc to yield the 1-(1H-indol-2-yl)ethanone 3.
References:
Kumar, Nag S.;Amandoron, Emily A.;Cherkasov, Artem;Brett Finlay;Gong, Huansheng;Jackson, Linda;Kaur, Sukhbir;Lian, Tian;Moreau, Anne;Labrière, Christophe;Reiner, Neil E.;See, Raymond H.;Strynadka, Natalie C.;Thorson, Lisa;Wong, Edwin W.Y.;Worrall, Liam;Zoraghi, Roya;Young, Robert N. [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 24,p. 7069 - 7082] Location in patent:supporting information