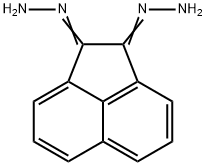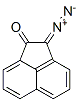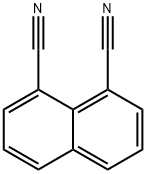
1,8-Dicyanonaphthalene synthesis
- Product Name:1,8-Dicyanonaphthalene
- CAS Number:5690-48-2
- Molecular formula:C12H6N2
- Molecular Weight:178.19
![8-Thia-7,9-diaza-cyclopenta[a]acenaphthylene 8,8-dioxide](/CAS/20180601/GIF/51485-73-5.gif)
51485-73-5
3 suppliers
inquiry

5690-48-2
3 suppliers
inquiry
Yield:5690-48-2 90.6%
Reaction Conditions:
at 180; under 0.15 Torr;Pyrolysis;
Steps:
7 Synthesis of 1,8-dicyanonaphthalene, 2
Acenaphtho[1,2-c][1,2,5]thiadiazole 8,8-dioxide (1) (2.04 g, 8.41 mmol) was pyrolyzed under vacuum (150 mTorr) at 180°C in a water-cooled sublimation apparatus overnight. The resulting solids were collected and resublimed under vacuum (150 mTorr) at 180 °C overnight to give the product as a pure yellow solid (1.36 g, 90.6% yield). 1H NMR (CDCl3, 499.74 MHz): δ 8.18 (d, J = 8.4 Hz, 2H), 8.13 (d, J = 8.4 Hz, 2H), 7.68 (t, J = 7.6 Hz, 2H). 13C NMR (CDCl3, 125.67 MHz): δ 138.00, 134.68, 133.55, 128.99, 126.84, 116.81, 109.05. IR (neat): 3061, 2227, 2215, 1586, 1571, 1512, 1372, 1360, 1230, 1218, 1174, 1111, 1092, 990, 970, 943, 828, 779, 761, 694, 666 cm-1. HR-DART/MS (m/z): [M+H]+ 179.0605 (found); C12H7N2 179.0609 (calcd).
References:
Bernstein, Karl J.;Do-Thanh, Chi-Linh;Penchoff, Deborah A.;Alan Cramer;Murdock, Christopher R.;Lu, Zheng;Harrison, Robert J.;Camden, Jon P.;Jenkins, David M. [Inorganica Chimica Acta,2014,vol. 421,p. 374 - 379]
84300-93-6
0 suppliers
inquiry

5690-48-2
3 suppliers
inquiry
84331-69-1
0 suppliers
inquiry

5690-48-2
3 suppliers
inquiry
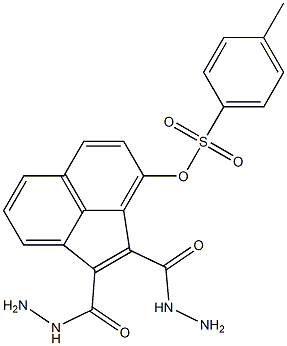
74340-05-9
0 suppliers
inquiry

5690-48-2
3 suppliers
inquiry