
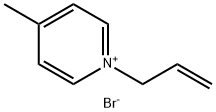
1-ALLYLPYRIDINIUM BROMIDE synthesis
- Product Name:1-ALLYLPYRIDINIUM BROMIDE
- CAS Number:10129-44-9
- Molecular formula:C8H10N.Br
- Molecular Weight:200.08
Yield: 99%
Reaction Conditions:
in acetonitrile at 70; for 31.5 h;
Steps:
General procedure: The new cholinesterase inhibitors (1-19; Figs. 2 and 3)were prepared via developed synthetic strategy [17-19]. Thesolution of amine/pyridine (5 mmol) and corresponding alkylatingagent (8 mmol) in MeCN (10 ml) was stirred at 70°C. The reaction mixture was cooled to the room temperatureand it was portioned with acetone (50 ml) and cooled in refrigerator(5°C) overnight. The crystalline or amorphouscrude product was collected by filtration, washed with acetone(320 ml) and crystalized from acetone. NMR, ESI-MSand elemental analysis determined the entity and purity of allprepared compounds
References:
Benek, Ondrej;Musilek, Kamil;Horova, Anna;Dohnal, Vlastimil;Dolezal, Rafael;Kuca, Kamil [Medicinal Chemistry,2015,vol. 11,# 1,p. 21 - 29]
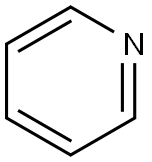
110-86-1
837 suppliers
$9.00/5g
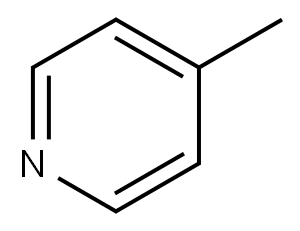
10129-54-1
3 suppliers
inquiry
108-89-4
320 suppliers
$15.00/25mL

10129-44-9
8 suppliers
inquiry
