
1-Amino-5,6,7,8-tetrahydroisoquinoline synthesis
- Product Name:1-Amino-5,6,7,8-tetrahydroisoquinoline
- CAS Number:75704-51-7
- Molecular formula:C9H12N2
- Molecular Weight:148.21
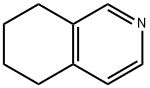
36556-06-6
125 suppliers
$45.00/250mg

75704-51-7
25 suppliers
$175.00/100mg
Yield:75704-51-7 46%
Reaction Conditions:
Stage #1: 5,6,7,8-tetrahydroisoquinolinewith sodium amide in N,N-dimethyl-formamide at 170; for 16 h;
Stage #2: with water;sodium hydroxide in N,N-dimethyl-formamide at 20;
Steps:
23.W.a
a. 5,6,7,8-Tetrahydroisoquinolin-1-amine To a solution of 5,6,7,8-tetrahydroisoquinoline (5.0 g, 37.6 mmol) in DMF (8 mL) was added sodium amide (2.55 g, 63.9 mmol). The resulting mixture was heated to 170° C. for 16 h and cooled to room temperature. To the mixture was added aqueous 2 N NaOH (50 mL). The mixture was extracted with DCM (100 mL*3), and the combined organic phase was dried over sodium sulfate and concentrated. The residue was purified by silica gel column chromatography (eluting with DCM/MeOH v/v 10:1) to give 5,6,7,8-tetrahydroisoquinolin-1-amine as a yellow solid (2.6 g, yield 46%). ESI MS: m/z 149 [M+H]+. 1H NMR (400 MHz, CDCl3): δ 7.80 (d, J=5.2 Hz, 1H), 6.44 (d, J=5.2 Hz, 1H), 4.34 (br, 2H), 2.67 (t, J=12 Hz, 2H), 2.39 (t, J=12 Hz, 2H), 1.88 (m, 2H), 1.78 (m, 2H).
References:
US2012/178748,2012,A1 Location in patent:Page/Page column 52
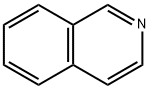
119-65-3
405 suppliers
$5.00/25g

75704-51-7
25 suppliers
$175.00/100mg