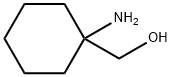
(1-aminocyclohexyl)methanol synthesis
- Product Name:(1-aminocyclohexyl)methanol
- CAS Number:4313-56-8
- Molecular formula:C7H15NO
- Molecular Weight:129.2

2756-85-6
224 suppliers
$20.00/5G

4313-56-8
38 suppliers
$72.00/100mg
Yield:4313-56-8 99%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 20;Inert atmosphere;Reflux;
Steps:
(l-Aminocyclohexyl)methanol.
(l-Aminocyclohexyl)methanol. 2M Lithium tetrahydroaluminate in tetrahydrofuran(80.0 mL, 160 mmol) was charged into a 500 mL 3 -necked round bottomed flask (oven-dried and cooled under argon) fitted with a magnetic stir bar and the solution was cooled to 0°C under argon. 1- Aminocyclohexanecarboxylic acid (7.64g, 53.3 mmol) is added portionwise over a period of 1 hour. At the end of the addition, the reaction mixture was diluted with tetrahydrofuran (60 mL), slowly warmed to room temperature, and then heated at reflux for 18 hours. The mixture was cooled to room temperature. The reaction mixture was further diluted with tetrahydrofuran (160 mL) and then cooled to 0°C. Saturated aqueous sodium carbonate (100 ml) was added very slowly keeping the internal temperature below 15°C. After the addition of the carbonate solution is complete, the ice bath was left to expire and the mixture slowly warmed to room temperature overnight. The reaction mixture was filtered thru a pad of Celite washing with ethyl acetate (400 mL). The solvent was removed in vacuo to afford a wet oil which was taken up in methylene chloride (300 mL) and dried over sodium sulfate. Filtration and concentration of the solvent in vacuo affords 6.89g (99% yield) of the desired product as a clear colorless oil. NMR (CDCls) 3.34 (s, 2H), 1.81 (bs, 3H), 1.51-1.32 (m, 10H); MS (ESI+) for C7Hi5NO m/z 130.0 (M+H)+ .
References:
WO2015/161287,2015,A1 Location in patent:Page/Page column 119-120
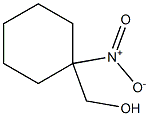
3719-52-6
2 suppliers
inquiry

4313-56-8
38 suppliers
$72.00/100mg
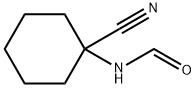
1125-02-6
5 suppliers
inquiry

4313-56-8
38 suppliers
$72.00/100mg

285124-16-5
1 suppliers
inquiry

4313-56-8
38 suppliers
$72.00/100mg
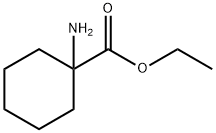
1664-34-2
21 suppliers
$88.00/500mg

4313-56-8
38 suppliers
$72.00/100mg