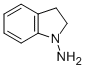
1-AMINOINDOLINE synthesis
- Product Name:1-AMINOINDOLINE
- CAS Number:7633-56-9
- Molecular formula:C8H10N2
- Molecular Weight:134.18
496-15-1
425 suppliers
$8.00/10g

7633-56-9
10 suppliers
inquiry
Yield:7633-56-9 37%
Reaction Conditions:
with potassium carbonate;hydroxylamine-O-sulfonic acid in water;acetonitrile;
Steps:
R.3 Synthesis of 1-aminoindoline
REFERENCE EXAMPLE 3: Synthesis of 1-aminoindoline In 40 ml of water was dissolved 2.76 g(20 mmol) of potassium carbonate thoroughly, and 10 ml of acetonitrile and 2.24 ml(20 mmol) of indoline were added thereto in this order. After stirring the reaction mixture for 10 min, 2.26 g(20 mmol) of hydroxylamine-O-sulfonic acid was added thereto. After stirring at room temperature for 1.5 hours, the reaction mixture was concentrated under the reduced pressure. The concentrate was extracted with dichloromethane and treated with anhydrous magnesium sulfate. After filtration, the filtrate was concentrated under the reduced pressure, and the resulting concentrate was subjected to chromatography over silica gel using dichloromethane as an eluent. The fractions were concentrated to give 0.99 g of the desired compound(37%). Rf=0.17 (in dichloromethane) NMR (DMSO-d6, δ) 2.73~3.06 (m, 2H, --CH2 --), 3.25~3.68 (m, 2H,NH2) 4.10~6.3 (br, 2H, N--CH2 --), 6.71~7.01 (m, 2H) 7.02~7.29 (m, 2H)
References:
US5336673,1994,A

7633-57-0
24 suppliers
inquiry

7633-56-9
10 suppliers
inquiry