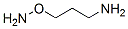
1-aminooxy-3-aminopropane synthesis
- Product Name:1-aminooxy-3-aminopropane
- CAS Number:98532-00-4
- Molecular formula:C3H10N2O
- Molecular Weight:0
To a solution of 35.0 gm (0.1 mole) of 3-N-phthalimidopropyloxy-N-phthalimide in 200 ml of DMF maintained at 70°C is added 20 gm (0.4 mole) of hydrazine hydrate. After allowing the solution to cool to room temperature over a 1-hr period, 300 ml of cold water is added and the pH of the solution is adjusted to pH 3 with hydrochloric acid. The precipitated phthalhydrazide is separated by filtration; the filtrate is evaporated between 40 and 50°C under reduced pressure. The residue is dissolved in 1 liter of methanol and the resulting solution is passed through a column containing 1 liter of IRA-400 ion-exchange resin (in OH" form, and previously treated with methanol). After the eluant is collected the column is rinsed with 1.5 liters of methanol. The eluant and methanol rinsings are combined and concentrated under reduced pressure to afford 3.0 gm (45%), b.p. 110-115°C/3 mm Hg. On redistillation 2 gm (30%) of purified product is isolated; b.p. 99°C/40 mm Hg; n25D 1.4615; d254 0.999.

894414-29-0
3 suppliers
inquiry
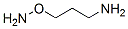
98532-00-4
3 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrazine hydrate in dichloromethane; for 2 h;Inert atmosphere;
Steps:
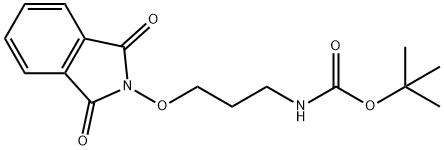
Synthesis of 3-(amino-oxy)propan-1-amine dihydrochloride (APA)
To a solution of tert-butyl {3-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)oxy]propyl}carbamate (compound 2 inFigure 2) (1.0 g, 3.13 mmol) in dry dichloromethane (15 ml), under an argon atmosphere was added hydrazine monohydrate (0.30 ml, 3.13 mmol) dropwise during 5 min. The solution turned into a suspension in 30 min.After 2 h, the white precipitate was filtered off and washed with cold dichloromethane (10 ml). The filtrate was concentrated in vacuo and the resulting residue was used without further purification. The residue was taken up in methanol (5 ml) and treated dropwise with hydrochloric acid (4.0 N in dioxane, 4.0 ml, 16.0 mmol). The mixture was stirred at room temperature, under an argon atmosphere, for 2 h and concentrated. The resulting residue was taken up in water (10 ml), washed with ethyl acetate (3 × 5 ml) and lyophilized to give the di-HCl salt of 3-(amino-oxy)propan-1-amine (APA) as a fine white powder (0.40 g, 78% yield for two steps).
References:
Zhou, X. Edward;Suino-Powell, Kelly;Schultz, Chad R.;Aleiwi, Bilal;Brunzelle, Joseph S.;Lamp, Jared;Vega, Irving E.;Ellsworth, Edmund;Bachmann, André S.;Melcher, Karsten [Biochemical Journal,2021,vol. 478,# 23,p. 4137 - 4149]
![2-[3-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)propoxy]-1H-isoindole-1,3(2H)-dione](/CAS/20180711/GIF/74651-79-9.gif)
74651-79-9
1 suppliers
inquiry
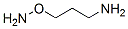
98532-00-4
3 suppliers
inquiry